NovaBay Pharmaceuticals, Inc. (AMEX: NBY)
8 Reasons Why NovaBay Pharmaceuticals, Inc. (AMEX: NBY) Could Be Poised For Significant Upside Potential in 2023.
LATEST NEWS
NovaBay Pharmaceuticals to Showcase its Full Line of Avenova Eyecare Products at the 126th Annual American Optometric Association Congress
Market Insights: The Global Ophthalmic Therapeutics Market, Psoriasis Treatment Market, and Cosmetics Market to Flourish, Valued at $68 Billion, $51 Billion, and $560 Billion by 2030 (16)(17)(18)
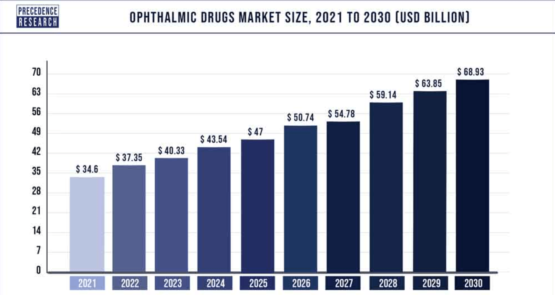

The global markets for ophthalmic therapeutics, psoriasis treatment, and cosmetics are experiencing rapid growth and offer lucrative opportunities. The ophthalmic therapeutics market, valued at $37.35 billion in 2022, is projected to reach approximately $68.93 billion by 2030. (16)
This market focuses on the development of therapeutics and formulations for treating various eye conditions such as glaucoma, cataracts, and macular degeneration. The growing investments in research and development activities, along with the increasing aging population, are driving market growth. (16)
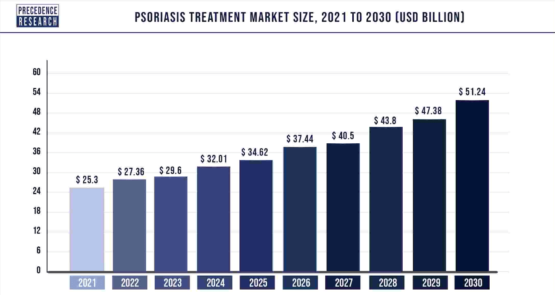

Similarly, the global psoriasis treatment market, valued at $25.3 billion in 2021, is estimated to reach around $51.24 billion by 2030. Psoriasis is an autoimmune inflammatory skin disorder characterized by symptoms like dryness, itching, bleeding, and local irritation. (17)
The market offers various treatments for different types of psoriasis, including psoriatic arthritis and plaque psoriasis. The increasing prevalence of psoriasis, particularly among the geriatric population, has led to a surge in demand for effective treatment options. (17)
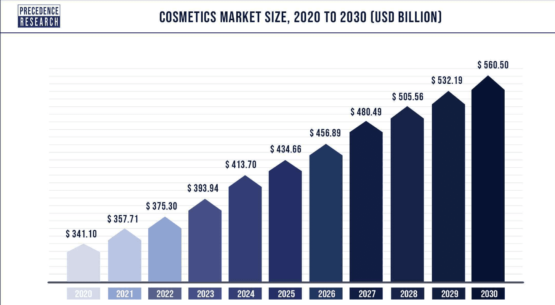

The cosmetics market, valued at $375.30 billion in 2022, is poised to reach approximately $560.50 billion by 2030. Factors such as shifting lifestyles, rising per capita income, urbanization, and the expanding middle-class consumer base drive this growth. (19)
The market has witnessed a rising demand for natural cosmetics products, fueled by consumer preferences for herbal and natural ingredients. (18)
Cosmetic skincare products are estimated to reach $185 billion by 2027. The skincare segment is growing faster than any other part of the beauty industry. (19)
Amidst these flourishing markets, NovaBay Pharmaceuticals, Inc. (AMEX: NBY) emerges as one company to keep a close eye on.
Could NovaBay Pharmaceuticals, Inc. (AMEX: NBY) Move 1,820% to the $12.00 Target Set By Ascendiant Capital Markets’ Director of Research, Edward Woo?(15)

Listen. Nothing is certain. But look at NovaBay Pharmaceuticals, Inc. (AMEX: NBY)’s weekly chart above and do a little technical analysis using StockCharts.com. You can see the following moving averages: (21)
As of 5/30/23, NovaBay Pharmaceuticals, Inc. (AMEX: NBY) has been trading in a weekly range of $0.64. (21)
However, what is truly fascinating are the moving averages that highlight the potential upside of NovaBay Pharmaceuticals, Inc. (AMEX: NBY).
The 50-day moving average for NovaBay Pharmaceuticals, Inc. (AMEX: NBY) is $3.762. A move back to this level could indicate a potential upside of 501.92%.
Similarly, the 100-day moving average for NovaBay Pharmaceuticals, Inc. (AMEX: NBY) is $9.316. If (NBY) was to move back to this level, it could indicate a potential upside of 1,390.56%.
Furthermore, the 200-day moving average for (NBY) is $18.228. A move back to this level could indicate a potential upside of 2,816.48%.
Additionally, considering the 52-Week Key Points from Barchart.com as of 5/30/2023, we observe that NovaBay Pharmaceuticals, Inc. (AMEX: NBY)’s 52-Week High is $12.635, which was reached on 8/8/2022. A move back to this level could indicate a potential upside of 1,921.6%. (21)
A move back to any of these levels is not certain, but the potential upside needs to be noted from current levels on 5/30/2023.
Maybe that’s why Ascendiant Capital Markets’ Director of Research, Edward Woo placed a $12.00 target on NovaBay Pharmaceuticals, Inc. (AMEX: NBY). (15)
"Ascendiant Capital Markets' Director of Research, Edward Woo Placed $12.00 Target on NovaBay Pharmaceuticals, Inc. (AMEX: NBY) (Source 15)"
Edward Woo, an experienced equity research analyst, serves as the Director of Research and Senior Analyst for Ascendiant Capital Markets, LLC. With over twenty years of industry expertise, including eight years at Ascendiant, Woo has established himself as a knowledgeable figure in the financial world. His insights and analysis have been sought after by major media outlets, and he has made appearances on CNBC and Bloomberg. Additionally, his opinions have been featured in reputable publications like Reuters, MarketWatch, and the Los Angeles Times. (22)
Throughout his career, Woo has been responsible for conducting thorough industry and company analyses, developing financial models, formulating recommendations, publishing reports, and monitoring performance. (22)
With his deep understanding of the industry and thorough financial modeling, Woo has set a target of $12.00 for NovaBay Pharmaceuticals, Inc. (AMEX: NBY). This projection indicates a potential upside of approximately 1,820% based on the (NBY) low of $.625 on 5/30/2023, according to Barchart.com. (1)(22)(15)
Warning: Do Not Ignore the Low Float of NovaBay Pharmaceuticals, Inc. (AMEX: NBY)


Low float stocks refer to the securities that remain after a company’s stock has been issued to its controlling investors — meaning there are relatively few shares for the public to buy. (3)
Market participants typically consider a float of 10-to-20 million shares as a low float. Some larger corporations have very high floats in the billions.
Companies with a low float frequently have a large portion of their equity held by controlling investors such as directors and employees, which leaves only a tiny percentage of the stock available for public trading.
Because low-float stocks have fewer shares available, market participants may have difficulty finding shares available.
That limited supply can cause dramatic swings if demand changes quickly. (3)
NovaBay Pharmaceuticals, Inc. (AMEX: NBY) has just under 1.57 million shares available for public trading as of 5/30/2023, according to Finviz.com. (2)
NovaBay Pharmaceuticals, Inc. (AMEX: NBY) is also considered a nano-cap.
In general, nano-cap companies have market capitalizations of less than $50 million. (2) Because nano-cap stocks are significantly smaller than mid-cap or large-cap companies, they have a higher potential to change valuation quickly.(9)
As of 5/30/23, NovaBay Pharmaceuticals, Inc. (AMEX: NBY) has a market cap of just around $1.9 million according to Finviz.com. (2)
Which is why things could get very interesting for NovaBay Pharmaceuticals, Inc. (AMEX: NBY).
IDENTIFYING THE OPPORTUNITY
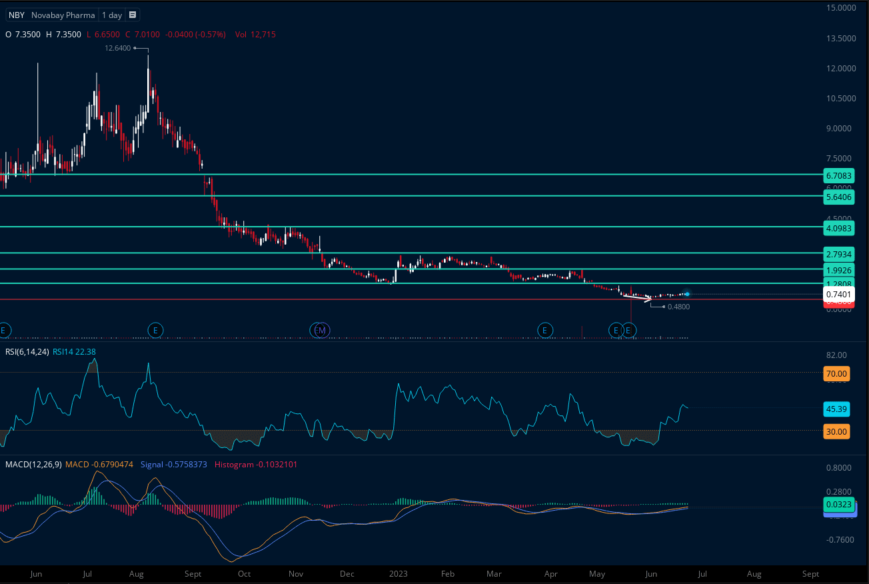

Low Float, High Potential
Supply and Demand are our best friends here, and they're telling us it's time
TARGETS
$1.28(+72.95%)
$1.99 (+168.88%)
$2.79 (+276.98%)
$4.09 (+452.63%)
BONUSES:
$5.64 (+662.06%)
$6.71 (+806.63%)
QVC Welcomes NovaBay Pharmaceuticals, Inc. (AMEX: NBY)'s Psoriasis Therapeutic Cream to its Network (23)


Image Source (24)
NovaBay Pharmaceuticals, Inc. (AMEX: NBY), a leading company in the development and commercialization of high-quality eyecare, skincare, and wound care products, has recently launched a new product in collaboration with DERMAdoctor. Chief Product Officer and board-certified dermatologist, Dr. Audrey Kunin, will introduce the innovative DERMAdoctor Comfort + Joy Psoriasis Therapeutic Moisturizing Cream with 3% Salicylic Acid as an on-air guest on the QVC network. This groundbreaking product aims to provide effective treatment and relief for individuals suffering from the symptoms of psoriasis. (23)
Psoriasis is a widespread condition that affects approximately 125 million people worldwide, with nearly 8 million individuals impacted in the United States alone. It can significantly impact the daily lives of those affected, causing itching, scaling, redness, flaking, and irritation. Understanding the challenges faced by psoriasis patients, NovaBay Pharmaceuticals and DERMAdoctor have developed this new moisturizing cream to alleviate these distressing symptoms. By combining the maximum-strength salicylic acid, known for its ability to control psoriasis symptoms and relieve itching, with other key ingredients such as ceramides, hyaluronic acid, and squalene, the cream helps restore essential moisture and maintain the skin’s barrier function.
The Psoriasis Therapeutic Moisturizing Cream with 3% Salicylic Acid will be exclusively available on QVC as a special offer, featuring two 8-fluid-ounce tubes for $52. Starting from July 31, 2023, the product will also be accessible on DERMAdoctor.com and Amazon for $46 per single tube. This advanced cream, which is steroid-free and fragrance-free, offers a clean formulation suitable for even the most sensitive skin, providing relief and preventing the recurrence of psoriasis symptoms. It aims to promote a soft, supple, and comfortable appearance, helping individuals regain their confidence and joy.
Dr. Audrey Kunin, a renowned figure in the skincare industry, brings her expertise as an author, clinician, educator, and television personality to the development of DERMAdoctor products. DERMAdoctor is committed to providing scientifically formulated skincare solutions that address common concerns overlooked by the beauty industry. The brand’s products are hypoallergenic, non-irritating, non-drying, pH balanced, and free of synthetic fragrances and dyes. By prioritizing measurable results and simplicity in clinical skin therapy, DERMAdoctor revolutionizes the skincare industry, ensuring effective solutions for individuals seeking relief from various skincare issues.
NovaBay Pharmaceuticals, Inc. (AMEX: NBY)’s collaboration with DERMAdoctor reflects its commitment to improving the lives of individuals through innovative skincare solutions. By leveraging the expertise of Dr. Audrey Kunin and her team, NovaBay Pharmaceuticals continues to lead the way in developing high-quality products that address unmet needs in the skincare market. The launch of the DERMAdoctor Comfort + Joy Psoriasis Therapeutic Moisturizing Cream with 3% Salicylic Acid on the QVC network marks another milestone in NovaBay Pharmaceuticals, Inc. (AMEX: NBY)’s mission to provide effective and accessible skincare options for those in need. (23)
As psoriasis continues to affect millions of individuals globally, NovaBay Pharmaceuticals’ partnership with DERMAdoctor demonstrates their dedication to improving the lives of those living with this challenging condition. Through the introduction of innovative products like the Psoriasis Therapeutic Moisturizing Cream, NovaBay Pharmaceuticals, Inc. (AMEX: NBY) remains at the forefront of skincare advancements, providing hope and relief for individuals seeking effective treatment options. (23)
On March 14, 2023, NovaBay Pharmaceuticals, Inc. (AMEX: NBY) announced a partnership with Eyeganics to sell OTC Organic Tears (0.2% organic glycerin) on Avenova.com and through Avenova’s physician-dispensed channel. Organic Tears has no additives or artificial chemicals and is the only lubricant eye drop certified by the USDA as 100% organic and preservative-free.
Organic Tears are formulated using only three ingredients – organic glycerin (oil from organic vegetables), salt, and water – and are extremely refreshing to dry eyes. A new technology allows for each bottle to dispense more than 250 drops of Organic Tears, providing a cost and convenience advantage over other preservative-free artificial tears found in single-use vials.
(15)
Dry eye is a multifaceted and complex condition that’s becoming increasingly common. We’ve now added another high-quality, scientifically formulated product to Avenova.com that further reinforces our website as a one-stop designation for customers seeking relief from the symptoms of dry eye,
said Justin Hall, NovaBay CEO. (10)
"As a practicing eye doctor, I talk to a lot of patients about what eye drops they use," said Eyeganics founder and CEO Dan Friederich, OD, FAAO.
"I developed this product with all-natural ingredients to address the needs of patients affected by some chemicals that may be found in other artificial tears. I’m delighted to partner on this opportunity and to associate Organic Tears with the high-quality products available on Avenova.com." (10)


NovaBay Pharmaceuticals, Inc. (AMEX: NBY) develops and sells scientifically created and clinically proven eye care and skincare products. NovaBay’s leading product, Avenova® Antimicrobial Lid & Lash Solution, is often prescribed by eyecare professionals for blepharitis and dry-eye disease and is also available directly to eye care consumers through online distribution channels such as Amazon. (10)


DERMAdoctor® offers more than 30 OTC dermatologist-developed skin care products through the DERMAdoctor website, well-known traditional and digital beauty retailers, and international distributors. (10) NovaBay also manufactures and sells effective, yet gentle and non-irritating wound care products. The PhaseOne® brand is distributed through commercial partners in the U.S. for professional use only, and the NeutroPhase® brand is distributed in China by Pioneer Pharma (Hong Kong) Company Ltd. (10)


NovaBay Pharmaceuticals, Inc. (AMEX: NBY) Launches New Antioxidant-Rich Oral Supplement to Comfort Dry Eyes and Promote Overall Eye Health(11)
On March 07, 2023, NovaBay Pharmaceuticals, Inc. (AMEX: NBY) announced the launch of OTC Avenova Eye Health Support oral supplement featuring a combination of MaquiBright®, a nutrient-rich, antioxidant-dense extract of the superfruit maqui berry, and high quality, natural triglyceride omega-3 oils to comfort dry eyes and support overall eye health. (11)
Avenova Eye Health Support is sold as 60 easy-to-swallow soft gel capsules (30-day supply) and is available at Amazon.com and Avenova.com for $41.95, and through optometrist offices participating in NovaBay’s physician-dispensed channel.
"Our eyes are under more stress than ever with increasing computer screen time, air pollution, and indoor heating and cooling, all of which can lead to dry eye and eye fatigue. In fact, up to 49 million Americans suffer from dry eyes and the global market for dry eye products is expected to grow at 5% annually, reaching nearly $9 billion by 2030"
said Justin Hall, NovaBay CEO. (11)
"We’re excited to offer a unique eye health supplement that has the potent maqui berry extract, which has three times the antioxidant level of blueberries and is found only in the forests of Patagonia. This product is unique in the marketplace and the clinical effects of the maqui berry extract are impressive."
Participants in a four-week, randomized, double-blind, placebo-controlled study on the effects of MaquiBright® extract showed an 89% increase in tear production and reported a 57% improvement in dry eye discomfort with Avenova Eye Health Support. Study results were published in the peer-reviewed Journal of Traditional and Complementary Medicine.
"Avenova Eye Health Support is another example of NovaBay being at the forefront of innovation in the eyecare category as we continue expanding our portfolio of evidence-led, differentiated, superior-quality, and highly effective eye care products,"
Hall added
Consumers and eyecare professionals alike now have another Avenova product to address the multifactorial and complex condition of dry eyes.
The oral supplement is the latest addition to Avenova’s portfolio of scientifically developed eye care products targeting the dry eye market.
With the strategic addition of an oral supplement, the Avenova brand now offers a best-in-class product for each step of the standard dry-eye treatment regimen: Avenova antimicrobial lid and lash spray for cleansing, lubricating eye drops for instant relief, a warm eye compress to soothe, a dietary supplement of omega-3 oils, and the i-Check to monitor physical eyelid health.
All Avenova products are available directly to consumers through online distribution channels such as Amazon and Avenova.com. (11)
NovaBay Pharmaceuticals, Inc. (AMEX: NBY) Expands Commercial and Collaborative Strategies in $30 Billion Wound Care Market (13)


On January 17, 2023, PhaseOne Health announced the expansion of its commercial program for PhaseOne Skin and Wound Care Cleanser through collaborative partnerships with innovative wound dressing manufacturers and key wound and burn care specialists in the United States. PhaseOne is formulated with NovaBay Pharmaceuticals, Inc. (AMEX: NBY)’s patented, stable hypochlorous acid. (13)
"We expect wound care to be a larger part of our business in 2023 and we are excited to partner with PhaseOne Health to grow our sales in the coming year, "
Said Justin Hall, CEO of NovaBay Pharmaceuticals. (13)
We are building on triple-digit percentage growth over the last few years by aligning with key opinion leaders and advisors to expand our commercial programs in 2023. Combining our past commercial success with recent results in new clinical applications positions us well to pursue synergistic business and protocol development with other companies and providers to address large market opportunities not only in wound care but in other surgical applications as well.
Kris Perkins, Managing Director of PhaseOne Health, further explained
PhaseOne is pure hypochlorous acid (HOCI), a part of the body’s natural immune system. HOCl is widely known as safe and effective for managing biofilm, bacteria, and fungi to promote the healing process. Naturally produced by the body’s white blood cells, HOCl was first synthesized by French scientist Antoine Jerome Balard in 1834 however its use in clinical applications has expanded greatly within the past 10 years.
While PhaseOne is primarily used for pre- and post-plastic surgery procedures, this coming year will bring an increased focus in the wound care market.
PhaseOne differentiates itself in the wound care market by utilizing NovaBay’s unique formulation of HOCI.
Through its proprietary manufacturing process and amber glass bottle, NovaBay Pharmaceuticals, Inc. (AMEX: NBY) ensures the safest, purest, most powerful HOCl wound cleanser on the market. Its effectiveness is independently documented in the study Comparative Antimicrobial Activity of Commercial Wound Care Solutions on Bacterial and Fungal Biofilms (Harriott, Ph.D. et al) published in the peer-reviewed journal Annals of Plastic Surgery.
The Comparative Antimicrobial Activity study is unique and compelling because of the large number of bacterial species evaluated. Even more significant is the large number of bacterial and fungal strains evaluated within each of these groups. This study documented the efficacy of HOCI in general for penetrating biofilm and killing numerous gram-negative and gram-positive bacteria as well as fungi. It also showed that when hypochlorous acid is stored in UV-resistant glass, such as Phase One, compared to plastic, there is a trend for faster action in penetrating biofilm and killing associated organisms. When dealing either with a surgical pocket or a wound, purity, and speed of action are more likely to benefit the clinical situation.
Said Jack Fisher, MD, Chief Medical Officer of PhaseOne Health. (13)
"Due to PhaseOne Health’s recent successes in the wound care market, we will be looking to expand our efforts." Mr. Perkins added, "This year we are excited to focus on partnering with organizations committed to providing more efficient, cost-effective healing solutions and therapies for wound and burn patients. We will also continue supporting independent studies that further explore the utilization of PhaseOne in other specializations like women’s health. We are looking forward to seeing PhaseOne Health continue to impact the wound care market and help to heal those in need." (13)
PhaseOne Health hypochlorous acid safely penetrates biofilm. Biofilm represents a significant barrier to wound healing. PhaseOne (HOCl) solution is effective at penetrating biofilm and mitigating its complications. PhaseOne is a broad-spectrum non-toxic and non-irritating solution and does not lead to antimicrobial resistance.
Based in Nashville, TN, PhaseOne Health licenses, markets, and distributes PhaseOne, a registered trademark of NovaBay Pharmaceuticals, Inc. (13)
The Global Wound Care Market on Pace to Reach $30 Billion by 2032 (14)
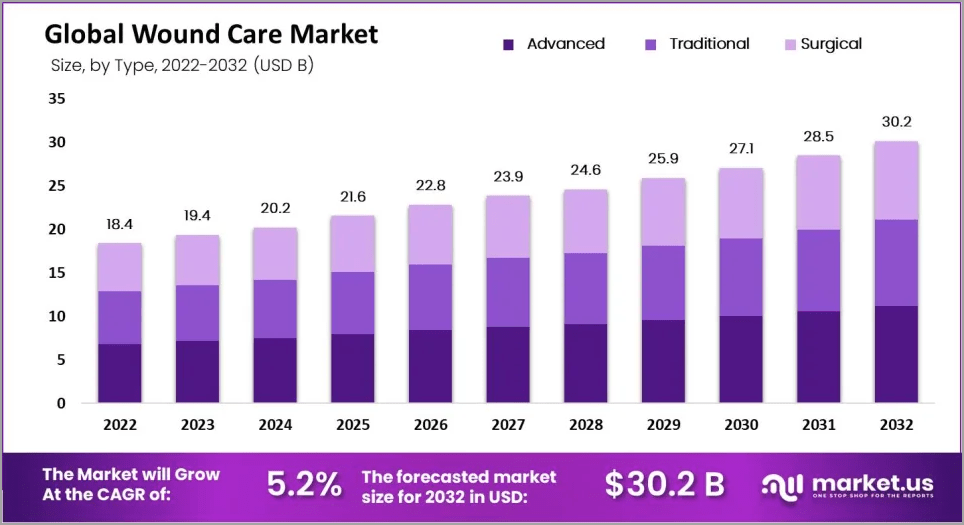

The global wound care market was worth USD 18.4 billion in 2022 and is projected to reach USD 30.2 billion by 2032, exhibiting a CAGR of 5.2% in the forecast period (2023-2032). (14)
It is estimated that healing will go through the anticipated stages of wound healing since a wound is a disturbance of the normal structure and function of skin and soft tissue. In addition, a chronic wound has physiological impairment.(14)
Wound care promotes healthy healing by concentrating on wounds that have injured or damaged the skin’s underlying structure.
Acute and chronic wounds, as well as any other healing or repair of injured human tissue, including injuries brought on by therapeutic radiation and mild burns, fall under the umbrella of the wound care industry, which expressly excludes the burning business. By providing adequate fluid balance, co-efficiency, and convenience for the patients and nursing staff, wound dressing aids in the removal of dead space and the prevention of bacterial growth. As a result of numerous technical developments, the global market for wound care has become the norm for treating both acute and chronic wounds. (14)
Factors affecting the growth of the Wound Care industry(14)
Surging diabetes levels that have an impact on wound healing: Diabetes has a negative impact on how effectively wounds heal. The prevalence of diabetes has dramatically increased over the years, along with cases of acute and chronic wounds.
Growing geriatric population: An increase in the number of the elderly population has also led to an increase in surgical procedures. The complexity of wounds has risen, particularly for infections, ulcerations, and other chronic wounds, as a result of increasing obesity rates and other health issues.
Product approvals by regulatory agencies are increasing: Government agencies are undertaking initiatives to introduce new products into the market for improved wound management. As a result, a rising number of product approvals is anticipated to fuel this market's further expansion.
Increasing Ambulatory Surgical Centers (ASCs): A surge in the number of Ambulatory Surgical Centers (ASCs) is anticipated to fuel the wound care industry. ASCs provide a range of treatments, including diagnostic tests, surgical treatment, and wellness checks. Ambulatory surgical facilities also do procedures pertaining to the Gastro-Intestinal (GI) system, orthopedics, urology, restorative, reconstructive, or alternative plastic surgery. Also, as they become more prevalent, ASCs provide affordable services.
NovaBay Pharmaceuticals, Inc. (AMEX: NBY) Announces $1 Million Order for NeutroPhase Skin and Wound Cleanser from China Pioneer Pharma Holdings. (source 25)
NovaBay Pharmaceuticals, Inc. (AMEX: NBY) has recently fulfilled a significant order for its NeutroPhase Skin and Wound Cleanser, totaling $1 million, for China Pioneer Pharma Holdings, Limited. This partnership with China Pioneer, a prominent importer, and marketer of branded pharmaceuticals and medical devices in China, demonstrates NovaBay’s growing presence in the international market. The order has been successfully fulfilled, and the revenue is expected to be recognized in the second quarter of 2023. (25)

NeutroPhase is a proprietary, FDA-cleared, 510(k) skin and wound cleanser containing pure hypochlorous acid in saline. Intended for use under the supervision of healthcare professionals, NeutroPhase has been cleared for cleansing and removal of foreign material, including microorganisms and debris, from wounds. (26)
NeutroPhase is a proprietary, FDA-cleared, 510(k) skin and wound cleanser containing pure hypochlorous acid in saline. Intended for use under the supervision of healthcare professionals, NeutroPhase has been cleared for cleansing and removal of foreign material, including microorganisms and debris, from wounds. (26)
NeutroPhase, developed by NovaBay Pharmaceuticals, is a highly effective and safe hypochlorous acid wound cleanser. It stands out in the market for its superior purity, power, and gentleness on the skin and new tissue. Unlike many other wound care products, NeutroPhase does not contain toxic chemicals, making it a preferred choice among healthcare professionals. The product is manufactured in the United States through a proprietary process that ensures large batch quantities are produced.
According to Justin Hall, the CEO of NovaBay Pharmaceuticals, NeutroPhase’s efficacy is unparalleled in the wound care market. It has the ability to kill bacteria that infect wounds, neutralize toxins that impede healing, and destroy healthy tissue. Additionally, NeutroPhase is non-toxic and can be safely used on any wound as frequently as needed. One of its unique advantages is its capacity to penetrate and eliminate biofilm, a major obstacle to wound healing. (25)
China Pioneer, led by Chairman Paul Li, expresses pride in the long-standing partnership with NovaBay and the opportunity to offer high-quality products to healthcare customers in China. With their dedication to importing advanced-technology pharmaceuticals and medical devices, China Pioneer aims to benefit patients across the country. (25)
In the United States, NovaBay manufactures a similar wound care product called PhaseOne under its PhaseOne Health brand. The effectiveness of PhaseOne has been independently documented in a study titled “Comparative Antimicrobial Activity of Commercial Wound Care Solutions on Bacterial and Fungal Biofilms,” published in the peer-reviewed journal Annals of Plastic Surgery.
The partnership between NovaBay Pharmaceuticals and China Pioneer Pharma Holdings, Limited highlights the global demand for innovative wound care solutions. As NovaBay continues to expand its presence and deliver effective products like NeutroPhase to international markets, it reinforces its position as a leader in the field of advanced wound care. (25)
Sources
- Source 1: https://www.barchart.com/stocks/quotes/NBY/price-history/historical
- Source 2: https://finviz.com/quote.ashx?t=NBY&p=d
- Source 3: https://www.sofi.com/learn/content/understanding-low-float-stocks/
- Source 4: https://www.investopedia.com/terms/n/nanocap.asp
- Source 5: https://s3.amazonaws.com/b2icontent.irpass.cc/2807/189210.pdf
- Source 6: https://www.verifiedmarketresearch.com/product/eye-care-products-market/
- Source 7: https://schrts.co/CSUqrZCC
- Source 8: https://public.ortex.com/wp-content/uploads/2022/04/1978109978-huge-scaled.jpg
- Source 9: https://www.benzinga.com/money/best-nano-cap-stocks
- Source 10: https://finance.yahoo.com/news/eyeganics-organic-tears-only-usda-105000743.html
- Source 11: https://finance.yahoo.com/news/novabay-pharmaceuticals-launches-avenova-eye-115000785.html
- Source 12: https://pbs.twimg.com/media/FrNDOEEWYAAKqnv?format=jpg&name=900×900
- Source 13: https://finance.yahoo.com/news/phaseone-health-novabay-pharmaceuticals-expand-115000665.html
- Source 14: https://finance.yahoo.com/news/wound-care-market-size-estimated-143500120.html
- Source 15: https://cdn.aelieve.com/5bdb71c4-nby-2023.04.02-q4-2022-earnings.pdf
- Source 16: https://www.precedenceresearch.com/ophthalmic-drugs-market
- Source 17: https://www.precedenceresearch.com/psoriasis-treatment-market
- Source 18: https://www.precedenceresearch.com/cosmetics-market
- Source 19: https://s3.amazonaws.com/b2icontent.irpass.cc/2807/190104.pdf
- Source 20: https://finance.yahoo.com/news/novabay-pharmaceuticals-reports-first-quarter-200500375.html
- Source 21: https://schrts.co/mZtfsPyX
- Source 22: https://ascendiant.com/team/edward-m-woo-cfa/
- Source 23: https://www.businesswire.com/news/home/20230421005008/en/NovaBay-Pharmaceuticals-Launches-DERMAdoctor%E2%80%99s-New-Comfort-Joy-Psoriasis-Therapeutic-Moisturizing-Cream-on-the-QVC%C2%AE-Network
- Source 24: https://scontent-lga3-2.xx.fbcdn.net/v/t39.30808-6/342377042_243681768183219_2409397133869954872_n.jpg?_nc_cat=107&ccb=1-7&_nc_sid=730e14&_nc_ohc=aDL38ldy1A8AX-eKGBN&_nc_ht=scontent-lga3-2.xx&oh=00_AfBkMLQJQPwFrw8V-FKDPzKo5HaYbei3nrU8UrL4H3PiyQ&oe=647B57E6
- Source 25: https://finance.yahoo.com/news/novabay-pharmaceuticals-fulfills-1-million-105000124.html
- Source 26: https://novabay.com/products/neutrophase/
- Source 27: https://finance.yahoo.com/news/novabay-pharmaceuticals-showcase-full-line-105000813.html
Disclaimer
Disclaimer
Stock Research Today is a project of Virtus Media Group LLC and intended solely for entertainment and informational purposes. This website / media webpage is owned, operated and edited by Virtus Media LLC. Any wording found on this website / media webpage or disclaimer referencing “I” or “we” or “our” or “Virtus Media” refers to Virtus Media LLC. This website / media webpage is a paid advertisement, not a recommendation nor an offer to buy or sell securities. By reading our website / media webpage you agree to the terms of this disclaimer, which are subject to change at any time. We are not registered or licensed in any jurisdiction whatsoever to provide investment or brokerage advice or anything of an advisory or consultancy nature and therefore are unqualified to give investment recommendations. Always do your own research and consult with a licensed investment professional before investing. This communication is never to be used as the basis for making investment decisions and is for entertainment and educational purposes only. At most, this communication should serve as a starting point to do your own research and consult with a licensed professional regarding the companies profiled and discussed. Companies with low price per share are speculative and carry a high degree of risk, so only invest what you can afford to lose. Consult your financial, investment and tax advisors to determine what financial and tax strategies may be right for you. Investor protection and other important information is available at https://www.sec.gov/.
By using our service, you agree not to hold our site, its editors, owners, or staff liable for any damages, financial or otherwise, that may occur due to any action you may take based on the information contained within or referred to from our website / media webpage. We do not advise any reader take any specific action. Losses can be larger than expected if the company experiences any problems with liquidity or wide spreads. Never invest purely based on our alerts. Gains mentioned in our website / media webpage may be based on end-of-day or intraday data.
This publication and its owners and affiliates may hold positions in the securities mentioned in our alerts, which we may sell at any time without notice to our subscribers, which may have a negative impact on share prices. If we own any shares we will list the information relevant to the stock and number of shares here. That information is only valid at the time it is published, and we do not undertake to update it.
Virtus Media’s business model is to receive financial compensation to promote public companies and to conduct investor relations advertising, marketing and publicly disseminate information, not limited to our websites, email, sms, push notifications, influencers, social media postings, ticker tags, press releases, online interviews, podcasts, videos, audio ads, banner ads, native ads, and responsive ads. This compensation is a major conflict of interest in our ability to be unbiased regarding the subject of our reports and communications. Therefore, this communication should be viewed as a commercial advertisement only. We have not investigated the background of the parties who hired us, or of the profiled companies. Any non-compensated alerts are purely for the purpose of expanding our database for the benefit of our future financially compensated investor relations efforts.
The third parties paying for our services, the profiled company, or their affiliates likely wish to liquidate shares of the profiled company at or near the time you receive this communication, which has the potential to impact share prices, possibly significantly. Frequently companies profiled in our alerts experience a large increase in volume and share price during the course of investor relations marketing, which may end as soon as the investor relations marketing ceases.
We do not guarantee the timeliness, accuracy, or completeness of the information on our website / media webpage. The information in our website / media webpage is believed to be accurate and correct, but has not been independently verified and is not guaranteed to be correct. The information is collected from public sources, such as the profiled company’s website and press releases, but is not researched or verified in any way whatsoever to ensure the publicly available information is correct. Furthermore, Virtus Media often employs independent contractor writers who may make errors when researching information and preparing these communications regarding profiled companies. Independent writers’ works are double-checked and verified before publication, but it is possible for errors or omissions to take place during editing of independent contractor writer’s communications regarding the profiled company(s). You should assume all information in all of our communications is incorrect until you personally verify the information, and again are encouraged to never invest based on the information contained in our written communications. Our emails may contain forward-looking statements, which are not guaranteed to materialize due to a variety of factors.
Compensation: Pursuant to an agreement between Virtus Media LLC and Lifewater Media, Virtus Media LLC has been hired by Lifewater Media LLC for a period beginning on 06/26/2023 and ending 06/29/2023 to publicly disseminate information about AMEX:NBY via digital communications. We have been paid fifteen thousand dollars USD.
