LATEST NEWS
HippoFi Ships International Orders and Expects Sales to Exceed $20 Million in the New Year
IDENTIFYING THE OPPORTUNITY
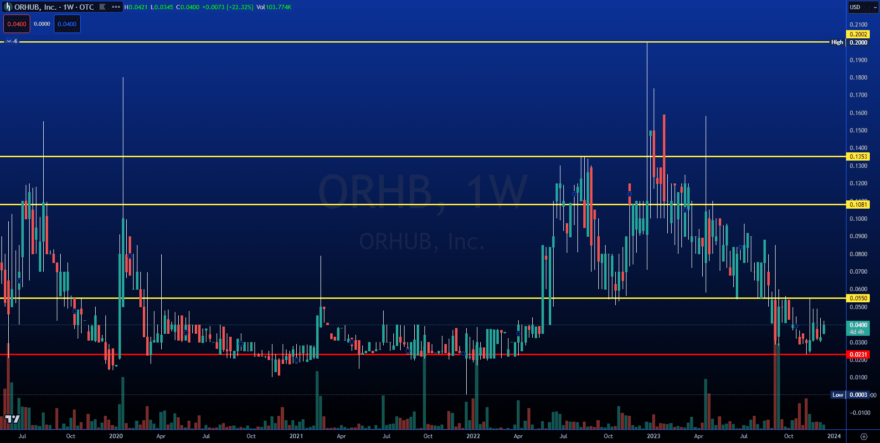

INSTITUTIONAL SUPPORT IS IN PLACE AND READY TO RUN
This is a strong historical level, and we are seeing increased volume. This is the opportunity we have been waiting for.
TARGETS
Target #1: $0.0550 (+37.50%)
Target #2: $0.1081 (+170.25%)
Target #3: $0.1353 (+238.25%)
Target #4: $0.2002 (+400.50%)
Support: $0.0231
HIPPOFI, INC.
(OTCPK: ORHB)
COMPANY OVERVIEW


The View From 30,000 feet
Numerous healthcare technology firms showcase impressive innovation, presenting promising technologies and approaches that, although beneficial, may take years to translate into sales. What sets HippoFi, Inc. (OTCPK: ORHB), formerly known as ORHub, apart is not only its innovative prowess in a crucial therapeutic sector but also the substantial growth experienced by its key subsidiary, PUR Biologics. This growth can be attributed not only to the excellence and diversity of its products but also to the robust network of distribution partnerships. Despite the remarkable achievements and the current undervaluation of the company, largely overlooked by the investing public, we believe that the relatively low valuation is a result of the early stage in the product sales cycle. The subdued average daily volume supports this viewpoint. However, as quarterly financial reports emerge and guidance is provided, there is a potential for these shares to attract aggressive accumulation.
An overview
HippoFi, Inc. (ORHB) is at the forefront of healthcare innovation, deploying cutting-edge solutions in lucrative markets such as Regenerative Therapeutics through its primary subsidiary, PUR Biologics. Acquired in 2022, PUR specializes in innovative biologic products and proprietary technologies for bone growth in surgical spine procedures, featuring a comprehensive portfolio encompassing patented bioactive cellular matrix compositions and advanced autologous cell therapies. Addressing multi-billion-dollar markets, PUR aims to regenerate cartilage, facilitate bone growth in surgical spine procedures, repair spinal discs, and alleviate back and central neuropathic pain.
HippoFi's strategic partnership with Precision Spine, a leader in the industry segment, has significantly boosted demand and sales for PUR products. Precision Spine, known for its extensive spine implant portfolio, now offers the complete PUR biologic line, providing a one-stop-shopping experience to its 207 sales distributors. This collaboration reaches 280 hospitals and approximately 340 spine surgeons conducting 800 surgeries monthly.
Beyond its core allografts and synthetics, PUR is actively developing novel, patented, and proprietary products related to its segment, anticipating future 510(k) submissions. These products incorporate cell therapy and contribute to pain management. ORHB maximizes its acquisition, in-licensing, and distribution strategies, expanding its portfolio with new FDA-approved offerings on a white-label basis and extending distribution channels for PUR.
Simultaneously, ORHB is in the process of creating innovative business management software to standardize processes at the point of surgical care, enhancing logistics and efficiency. While PUR remains the current revenue driver, the upcoming HippoFi-Pay™ division, set to launch in the FY24E fiscal year starting July 2023, will tap into the same target market and channels. This Software as a Service (SaaS)-based approach is expected to yield significantly higher gross margins for ORHB and potentially make a substantial impact in the calendar year 2024.
Financials
The Company did not record substantial revenue from fiscal year 2022 through the first six months of fiscal year 2023E, which concluded in December 2022. During this timeframe, ORHB focused on research and development activities and underwent a strategic shift in its model, particularly following the acquisition of PUR Biologics in late 2022. Once the acquisition was finalized, and relationships with Precision Spine and others were solidified, we believe that PUR commenced meaningful product sales in the March 2023 quarter. Consequently, our revenue forecast for fiscal year 2023E (ending June 2023) is $2.9 million, reflecting only two quarters of business.
Looking forward, we anticipate revenues of $28 million for fiscal year 2024E and $75 million in fiscal year 2025E. This growth is expected to be propelled by existing product lines and relationships, in addition to the timing of a projected 510(k) product and other technologies obtained through licensing agreements. If our projections hold true, the Compound Annual Growth Rate (CAGR) for ORHB from fiscal year 2023 to fiscal year 2025 would be 195%. It is noteworthy that we estimate the potential generation of significant operating income starting in fiscal year 2024, with a projected $2.7 million in this line item, resulting in a 9.7% operating margin. For fiscal year 2025E, we project an operating income and margin of $15.3 million and 20.4%, respectively.
Valuation
Our price target for the next 12 months is $0.37, calculated at 4.0 times the projected $75 million revenue for the fiscal year 2025E ending in June 2025. In the Peer Group analysis in Table III, we observe that the peer group has an average forward 2024 price/revenue multiple of 4.0x, aligning our target with this benchmark. Despite using a revenue figure that is six months beyond the peer group's timeline, we assert that this is justifiable due to ORHB's significantly higher revenue growth rate. It can also be argued that ORHB may merit trading at a premium to the price/revenue multiple. Consequently, employing a 5x price/revenue multiple and a 25x P/E on our fiscal year 2025E revenue and net income forecasts yields a $0.47 price target, which is consistent with metrics observed among leading players in the segment. Consequently, we believe there is potential for substantial future upside to our target.
HIPPOFI, INC.
(OTCPK: ORHB)
A BIOLOGICS PRIMER


The American Academy of Orthopaedic Surgeons (AAOS) defines the industry segment as follows:
"Orthobiologics are substances utilized by orthopaedic surgeons to enhance the healing of injuries, accelerating the recovery of fractured bones and injured muscles, tendons, and ligaments. These products are derived from natural substances found in the body, and when employed in higher concentrations, they have the potential to expedite the healing process."
Over the past 15-20 years, the development and utilization of biologics have seen significant growth, driven in part by advancements in stem cell research. Generally, orthopedic biologics are treatments that are isolated or derived from natural sources like human or animal stem cells, plasma, or tissues through innovative technologies. An example of a well-known biologic treatment, once widely publicized, involves injecting platelet-rich plasma (PRP). In this procedure, blood heavily concentrated with platelets from either the patient or a donor is injected into a affected joint.
Market Trends
Notably, a convergence of factors is propelling the expansion of this market. These factors encompass:
- Progress in healthcare, particularly in the realm of bone growth stimulation.
- Escalating instances of orthopedic disorders resulting in fractures.
- An aging population contributing to the market's growth.
- A surge in sports-related injuries.
- Increasing acceptance and use of orthobiologics materials.
- A heightened demand for outpatient surgical procedures.
Growth factors play a crucial role in cell signaling during the healing process, facilitating cellular functions. They are utilized in combination with bone grafts to promote healing. Stem cells, previously associated with limited data and safety information, particularly in areas requiring repair, are now gaining wider acceptance, particularly in the case of autologous bone marrow cells, an area where the Company specializes.
In the realm of orthopedic surgery, there is currently a lack of widely used products for cartilage repair, bone healing enhancement, and reduction of non-unions. The standard of care for such injuries involves maintaining the repair through the application of casts, traction, or fixation with plates, screws, or other implants, whether external or internal. The increasing utilization of biologics is anticipated to transform this segment into a market opportunity worth tens of billions in the next five years. Furthermore, as the shift towards biologics continues, a diversified company like PUR is uniquely positioned to emerge as a leader in the market.
BEHIND THE SCENES
HIPPOFI, INC.
(OTCPK: ORHB)
THE HIPPOFI LEADERSHIP TEAM
Christopher Wiggins, Chairman, Chief Executive Officer
Christopher is serial entrepreneur with over 20 years in C-level leadership and strategic negotiations. He has built multiple successful companies from the ground-up while creating synergistic partnerships that drive value. Christopher has served as Founder, Chairman and Chief Executive Officer of HippoFi, Inc. (formerly ORHub) since 2020 and has also been President and Founder Katalyst Medical, LLC since 2019. He has extensive experience both in corporate and distribution roles with significant medical device manufacturers like Medtronic, Smith & Nephew, Globus Medical, Integra, Precision Spine, Baxter, Zimmer Biomet, and others. He was also a Co-founder of PUR Biologics and from 2013-2022 and served as Co-founder/Inventor of Adaptive Biologix from 2014-2017, prior to its acquisition by Histogen, Inc. (NASDAQ - HSTO). Christopher received a Bachelor of Arts degree from Concordia University, Irvine and an MBA from Pepperdine University.
Ryan Fernan, Head of PUR Biologics
Mr. Fernan, brings direct access to a broad industry network with a proven track record of over 18 years in the medical device and biotechnology industry. Recognized as a frontrunner in sales and product development, Mr. Fernan's career included leadership roles associated with Johnson&Johnson/DePuy Spine, and then Zimmer/Biomet. With a strong entrepreneurial drive, Mr. Fernan also founded OC Surgical, Inc., which became the largest Actifuse distributor in the United States from UK-based orthobiologics company, ApaTech. His successes with Actifuse largely contributed to its purchase by Baxter (NYSE: BAX) for approx. $330M in 2010. Mr. Fernan went on to found PUR Biologics, quickly developing technologies from concept, through pre-clinical trials, to large animal trials in collaboration with the University of Colorado and UCSD, and into initial FDA human clinical trial discussions. These first- generation technologies were negotiated into a successful sale by Mr. Fernan to Histogen, Inc. (NASDAQ: HSTO), while he continued directing the licensing and development of the next generation of cell derived extracellular matrix technologies.
Colton Melby, Executive, Public Company Advisor
A seasoned C-level executive, Board member, entrepreneur and investor with over thirty years of leadership and operational experience with innovative private and public companies. In aggregate, he has taken four companies onto public exchanges with a combined market capitalization of over $1.5B during his tenure. He has served as an advisor to HippoFi since 2022 and its Chairman and CEO of ORHub, Inc. from 2006-2020. As the primary investor of Waytronx, Colton led the purchase of CUI and up-listed to NASDAQ. He played a critical role in doubling sales and market value appreciation to over $300M while successfully raising over $70M from broker/dealers. He is also the Vice-Chairman and former CEO of CEO Quest Resource Holding Corp (NASDAQ:QRHC) from 2012 – 2014. He led Earth911, Inc. public with a merger into QRHC growing the market cap from $20M to over $450M. From 2001-2008 he served as President and COO, BOARD MEMBER of Smith & Wesson. He as the sole financier in the acquisition of Smith & Wesson from Tompkins PLC by Saf-T Hammer, growing the market cap from $70M to over $1B, up-listing to the AMEX and NASDAQ. He has also served as the CEO of Metal-Form, Inc. from 1987 – 1999 where he led the precision aircraft parts manufacturer from a backlog of $30M to over $500M as a prime supplier to Boeing and Bombardier. Under his leadership, it Metal-Form was named manufacturer of the Year 3x by Boeing and 2x by Bombardier. He later negotiated the sale of the company to a large private equity firm in New York.
Sources
- Source 1: https://www.goldmanresearch.com/202303281368/Opportunity-Research/pending-merger-could-drive-nyse-players-market-cap-higher.html
- Source 2: https://www.drpjournal.com/16637-2/
- Source 3: https://finance.yahoo.com/news/hippofi-ships-international-orders-expects-100000343.html?.tsrc=fin-srch
- Source 4: https://finance.yahoo.com/quote/ORHB?p=ORHB&.tsrc=fin-srch
- Source 5: https://finance.yahoo.com/quote/ORHB/
- Source 6: https://www.hippofi.info/
- Source 7: https://purbiologics.com/
- Source 8: https://purbiologics.com/#products
- Source 9: https://purbiologics.com/?page_id=777#brochure
- Source 10: https://www.accesswire.com/viewarticle.aspx?id=753645
- Source 11: https://orthospinenews.com/2023/04/27/pur-biologics-expands-sales-network-by-15-adding-32-new-distributors/
Disclaimer
Disclaimer
Stock Research Today is a project of Virtus Media Group LLC and intended solely for entertainment and informational purposes. This website / media webpage is owned, operated and edited by Virtus Media Group LLC. Any wording found on this website / media webpage or disclaimer referencing “I” or “we” or “our” or “Virtus Media” refers to Virtus Media Group LLC. This website / media webpage is a paid advertisement, not a recommendation nor an offer to buy or sell securities. By reading our website / media webpage you agree to the terms of this disclaimer, which are subject to change at any time. We are not registered or licensed in any jurisdiction whatsoever to provide investment or brokerage advice or anything of an advisory or consultancy nature and therefore are unqualified to give investment recommendations. Always do your own research and consult with a licensed investment professional before investing. This communication is never to be used as the basis for making investment decisions and is for entertainment and educational purposes only. At most, this communication should serve as a starting point to do your own research and consult with a licensed professional regarding the companies profiled and discussed. Companies with low price per share are speculative and carry a high degree of risk, so only invest what you can afford to lose. Consult your financial, investment and tax advisors to determine what financial and tax strategies may be right for you. Investor protection and other important information is available at https://www.sec.gov/.
By using our service, you agree not to hold our site, its editors, owners, or staff liable for any damages, financial or otherwise, that may occur due to any action you may take based on the information contained within or referred to from our website / media webpage. We do not advise any reader take any specific action. Losses can be larger than expected if the company experiences any problems with liquidity or wide spreads. Never invest purely based on our alerts. Gains mentioned in our website / media webpage may be based on end-of-day or intraday data.
This publication and its owners and affiliates may hold positions in the securities mentioned in our alerts, which we may sell at any time without notice to our subscribers, which may have a negative impact on share prices. If we own any shares we will list the information relevant to the stock and number of shares here. That information is only valid at the time it is published, and we do not undertake to update it.
Virtus Media’s business model is to receive financial compensation to promote public companies and to conduct investor relations advertising, marketing and publicly disseminate information, not limited to our websites, email, sms, push notifications, influencers, social media postings, ticker tags, press releases, online interviews, podcasts, videos, audio ads, banner ads, native ads, and responsive ads. This compensation is a major conflict of interest in our ability to be unbiased regarding the subject of our reports and communications. Therefore, this communication should be viewed as a commercial advertisement only. We have not investigated the background of the parties who hired us, or of the profiled companies. Any non-compensated alerts are purely for the purpose of expanding our database for the benefit of our future financially compensated investor relations efforts.
The third parties paying for our services, the profiled company, or their affiliates likely wish to liquidate shares of the profiled company at or near the time you receive this communication, which has the potential to impact share prices, possibly significantly. Frequently companies profiled in our alerts experience a large increase in volume and share price during the course of investor relations marketing, which may end as soon as the investor relations marketing ceases.
We do not guarantee the timeliness, accuracy, or completeness of the information on our website / media webpage. The information in our website / media webpage is believed to be accurate and correct, but has not been independently verified and is not guaranteed to be correct. The information is collected from public sources, such as the profiled company’s website and press releases, but is not researched or verified in any way whatsoever to ensure the publicly available information is correct. Furthermore, Virtus Media often employs independent contractor writers who may make errors when researching information and preparing these communications regarding profiled companies. Independent writers’ works are double-checked and verified before publication, but it is possible for errors or omissions to take place during editing of independent contractor writer’s communications regarding the profiled company(s). You should assume all information in all of our communications is incorrect until you personally verify the information, and again are encouraged to never invest based on the information contained in our written communications. Our emails may contain forward-looking statements, which are not guaranteed to materialize due to a variety of factors.
Compensation: Pursuant to an agreement between Virtus Media Group LLC and Cambridge Consultants Inc, Virtus Media Group LLC has been hired by Cambridge Consultants Inc for a period beginning on 12/12/2023 and ending 12/16/2023 to publicly disseminate information about OTCPK: ORHB via digital communications. We have been paid four thousand dollars USD.