

CSE: HUGE
NASDAQ: HUGE


Today there isn't a cure for MS.
Today there isn't a cure for Depression.
FSD Pharma is working to change that.
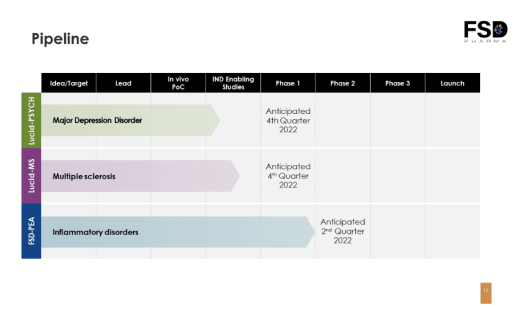
Pipeline of Clinical Candidates Addressing Multibillion-Dollar Markets
FSD Pharma is focused on innovative biotech space to bring advanced neurodegenerative and mental health treatments to market.
They are rooted in world-class science and research-based advancements.
Lucid Psych
A psychoactive molecule for the treatment of neuropsychiatric disorders currently undergoing IND-enabling studies.
Lucid-MS
A patented neuroprotective NCE shown to prevent and reverse myelin degradation, the underlying cause of multiple sclerosis, in preclinical models. Currently undergoing IND enabling studies.
FSD-PEA
A proprietary anti-inflammatory product completed phase-1 clinical trials with excellent safety profile, and is under evaluation for a suitable indication to launch phase-2 clinical trials.
Acquisition Of Lucid Psycheceuticals Inc. “Lucid”
With this transaction comes exciting new additions to their biotechnology pipeline, representing what we believe to be a major step toward fulfilling their mission of the most promising treatments for TOTAL BRAIN HEALTH.
Working with excellent minds in medicine and science at the University Health Network (UHN), the largest health research organization in Canada, they are focused on developing cutting-edge therapies for some of the hardest-to-treat diseases.
If you have a loved one or friend suffering with MS, you know the challenges they face today with current treatments and medicine. Similarly, if you are close to someone struggling with Depression, you know how hard it can be for them and their caregivers to find effective therapies. Even worse, MS can often cause Depression and Anxiety.
With this transaction comes exciting new additions to their biotechnology pipeline, representing what we believe to be a major step toward fulfilling their mission of the most promising treatments for TOTAL BRAIN HEALTH.
Working with excellent minds in medicine and science at the University Health Network (UHN), the largest health research organization in Canada, they are focused on developing cutting-edge therapies for some of the hardest-to-treat diseases.
If you have a loved one or friend suffering with MS, you know the challenges they face today with current treatments and medicine. Similarly, if you are close to someone struggling with Depression, you know how hard it can be for them and their caregivers to find effective therapies. Even worse, MS can often cause Depression and Anxiety.
How Lucid Acquisition Immediately Expands FSD-PEA Biotech Pipeline
Lucid has developed two unique drug candidates, which are complementary to our existing FSD-PEA (also known as FSD-201) drug candidate:
Lucid0MS for the treatment of MS.
LUCID-Psych targeting Depression.
Lucid-MS is a patented new chemical entity (meaning it is new and has never been used before) for MS that was developed over many years by doctors, scientists and researchers at the UHN. Lucid recently exclusively licensed Lucid-MS from the UHN.

Why Is This Important?
FSD Pharma's team believes all approved MS drugs today have been designed to target the immune system in one way or another because the general view in the medical world has been that MS is as autoimmune disease.
The Lucid scientific team thinks differently.
They believe that therapeutic applications involving Lucid-MS could be disease modifying by possibly reversing and protecting neurodegeneration without interacting with the immune system.
What Does This Mean?
Typically, a person with MS will lose control of their muscles over time (due to neurodegeneration), often making it difficult to maintain their balance when walking and/or performing delicate tasks with their hands.
This team believes Lucid-MS could be complementary to existing MS therapies, assisting people in a life-changing way by regaining the function they may have lost.
Beyond Lucid
The acquisition of Lucid not only brought new research and products, but a highly capable clinical research and development team with access to UHN research.
However, FSD doesn't plan on stopping here.
As excited as they are about this deal, company leadership will continue to search for addition unique investment and acquisition opportunities on the leading edge of medicine.
You can track their progress by following them on social media and keeping up with their website. Links provided below.

Expert Research and Clinical Team
An experienced and diverse multidisciplinary team guides the FSD Pharma mission. Learn more about their leadership and clinical staff here.
The Psychedelics Market Opportunity (Lucid-PSYCH)
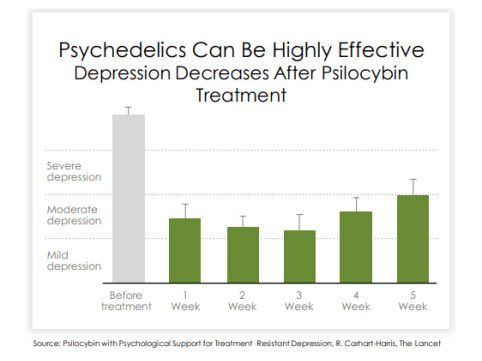
The FSD Pharma team views psychedelics as a new, underutilized vehicle in the fight against mental illnesses such as depression.
LUCID-Psych is not LSD, MDMA, Ketamine, Psilocybin, nor DMT. While it is a psychedelic-based medicine, LUCID-Psych was discovered using a state-of-the-art AI screening system and has been identified by the Lucid team for accelerated development due to its unique pharmaceutical properties and treatment potential in depression.
This new approach will be unveiled in the coming months, when the team is ready to submit their Investigational New Drug Application to the FDA.
Check out a few points below to get an idea of the implications this research could have on the biomed market.
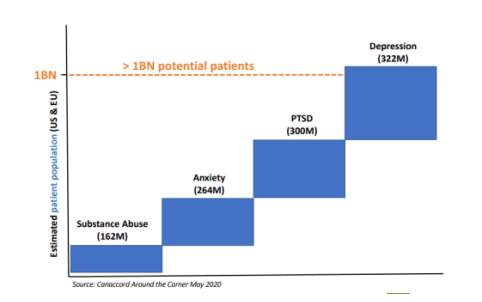
Growing bodies of research suggests psychedelics have the potential to treat a variety of
neuropsychiatric disorders such as PTSD, depression, specifically complex TRD and MDD,
and anxiety.
- FDA approval of SPRAVATO® (esketamine) for treatment-resistant depression
- Promising Ph2 clinical data on MDMA-assisted psychotherapy for PTSD treatment
- Expanding pipeline of psychedelic-based therapies
Millions of people suffer from mental illness each year;
representing a multi-billion dollar opportunity
FSD sees a significant opportunity in becoming an early mover in developing psychedelic assets.
The global anxiety disorders and depression treatment market size was valued at US$15.2B in 2015
Psychedelics market for mental Illness:
CAGR of 16.3% during 2020–27 and expected to reach US$6.9 Billion by 2027 from $2.0 Billion in 2019

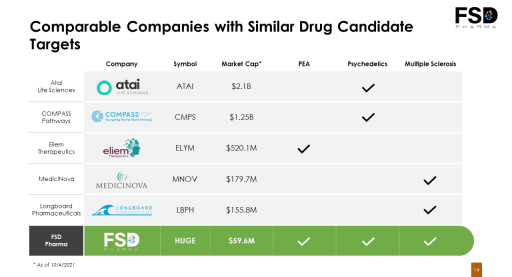
FSD Pharma Differentiation (Lucid-PSYCH)
Lucid-PSYCH | |
|---|---|
Development Stage | Preclinical |
Target Condition | Mental health and neurodegenerative conditions |
Proprietary Molecules | Yes |
Time To Market | 5-8 Years |
IP / Innovation | Yes |
Market Access Strategy | Multiple Entry Points |

Strong Financial Position
With $54.1 Million in current assets on the balance sheet, FSD continues to allocate funds to furthering research in the treatment of MS and Depression, two major markets within the medical field.
Cash Position: USD $43.2M
Over USD $54.1 Million Current Assets On The Balance Sheet As Of June 30, 2021
Capitalization Table:
Outstanding | |
|---|---|
Class A Shares | 72 |
Class B Shares | 40,557,896 |
Options | 3,314,810 |
Warrants | 6,749,109 |
*As of September 30th, 2021


Over USD $54.1 Million
current assets on the balance sheet as of
June 30th, 2021


Disclaimer
Stock Research Today is a project of Alan & Company Marketing LLC and intended solely for entertainment and informational purposes. Consult your financial, investment and tax advisors to determine what financial and tax strategies may be right for you. Investor protection and other important information is available at https://www.sec.gov/. This website / media webpage is owned, operated and edited by Alan & Company Marketing LLC. Any wording found on this website / media webpage or disclaimer referencing “I” or “we” or “our” or “Alan & Co” refers to Alan & Company Marketing LLC. This website / media webpage is a paid advertisement, not a recommendation nor an offer to buy or sell securities. Our business model is to be financially compensated to market and promote small public companies. By reading our website / media webpage you agree to the terms of our disclaimer, which are subject to change at any time. We are not registered or licensed in any jurisdiction whatsoever to provide investing advice or anything of an advisory or consultancy nature and therefore are unqualified to give investment recommendations. Always do your own research and consult with a licensed investment professional before investing. This communication is never to be used as the basis for making investment decisions and is for entertainment purposes only. At most, this communication should serve only as a starting point to do your own research and consult with a licensed professional regarding the companies profiled and discussed. Conduct your own research. Companies with low price per share are speculative and carry a high degree of risk, so only invest what you can afford to lose. By using our service you agree not to hold our site, its editor’s, owners, or staff liable for any damages, financial or otherwise, that may occur due to any action you may take based on the information contained within our website / media webpage .We do not advise any reader take any specific action. Losses can be larger than expected if the company experiences any problems with liquidity or wide spreads. Our website / media webpage are for entertainment purposes only. Never invest purely based on our alerts. Gains mentioned in our website / media webpage may be based on end-of-day or intraday data. This publication and their owners and affiliates may hold positions in the securities mentioned in our alerts, which we may sell at any time without notice to our subscribers, which may have a negative impact on share prices. If we own any shares we will list the information relevant to the stock and number of shares here. Alan & Co business model is to receive financial compensation to promote public companies. To conduct investor relations advertising, marketing and publicly disseminate information not limited to our websites, email, sms, push notifications, influencers, social media postings, ticker tags, press releases, online interviews, podcasts, videos, audio ads, banner ads, native ads, responsive ads. This compensation is a major conflict of interest in our ability to be unbiased regarding. Therefore, this communication should be viewed as a commercial advertisement only. We have not investigated the background of the hiring third party or parties. The third party, profiled company, or their affiliates likely wish to liquidate shares of the profiled company at or near the time you receive this communication, which has the potential to hurt share prices. Any non-compensated alerts are purely for the purpose of expanding our database for the benefit of our future financially compensated investor relations efforts. Frequently companies profiled in our alerts may experience a large increase in volume and share price during the course of investor relations marketing, which may end as soon as the investor relations marketing ceases. Our emails may contain forward-looking statements, which are not guaranteed to materialize due to a variety of factors.
We do not guarantee the timeliness, accuracy, or completeness of the information on our website / media webpage. The information in our website / media webpage is believed to be accurate and correct, but has not been independently verified and is not guaranteed to be correct. The information is collected from public sources, such as the profiled company’s website and press releases, but is not researched or verified in any way whatsoever to ensure the publicly available information is correct. Furthermore, Alan & Co. often employs independent contractor writers who may make errors when researching information and preparing these communications regarding profiled companies. Independent writers’ works are double-checked and verified before publication, but it is certainly possible for errors or omissions to take place during editing of independent contractor writer’s communications regarding the profiled company(s). You should assume all information in all of our communications is incorrect until you personally verify the information, and again are encouraged to never invest based on the information contained in our written communications. The information in our disclaimers is subject to change at any time without notice. Social media outlet compensation - Pursuant to an agreement between Alan & Company Marketing LLC and Dadmin capital LLC, Alan & Company Marketing LLC has hired Dadmin capital LLC for a period beginning on 12/2/2021 and ending after 5 business days to publicly disseminate information about (NASDAQ/CSE: HUGE) via digital communications. We have paid this Dadmin capital LLC Seven Thousand Five Hundred USD via ACH Bank Transfer. Compensation - Pursuant to an agreement between Alan & Company Marketing LLC and West Coast Media LLC, Alan & Company Marketing LLC has been hired for a period beginning on 12/02/2021 and ending after 10 business days to publicly disseminate information about (NASDAQ/CSE:HUGE) via digital communications. We have been paid twenty five thousand dollars USD via ACH Bank Transfer.






