RenovoRx: Revolutionizing Cancer Treatment with Groundbreaking Therapies and a $1B Market Opportunity
RenovoRx leverages its innovative TAMP™ platform to deliver life-saving therapies with significant survival benefits and reduced side effects, positioning itself as a leader in addressing high unmet medical needs.
About RenovoRx
RenovoRx is a late-clinical stage biopharmaceutical company dedicated to developing innovative targeted combination therapies for the treatment of hard-to-treat cancers. The company's flagship technology, the Trans-Arterial Micro-Perfusion (TAMP™) platform, is designed to enhance the therapeutic index of chemotherapeutic agents by delivering higher concentrations of the drug directly to the tumor site while minimizing systemic exposure and associated side effects. RenovoRx’s lead product candidate, RenovoGem™, is currently undergoing a pivotal Phase III clinical trial for locally advanced pancreatic cancer (LAPC). Interim results from this trial have demonstrated significant improvements in overall survival and a substantial reduction in adverse events, highlighting the potential of RenovoGem™ to set a new standard in cancer treatment.
Founded in 2009 and headquartered in Los Altos, California, RenovoRx has garnered significant industry recognition, including FDA Orphan Drug Designation for RenovoGem™ in the treatment of pancreatic and bile duct cancers. The company's pipeline is robust, with additional indications for RenovoGem™ under investigation, including bile duct cancer and glioblastoma. By leveraging its proprietary TAMP™ platform, RenovoRx aims to address the high unmet medical needs of patients with solid tumors that are difficult to treat with conventional therapies. With a strong leadership team and a strategic focus on expanding its innovative therapy platform, RenovoRx is well-positioned to make a meaningful impact on the future of cancer treatment.
LATEST NEWS
RenovoRx Announces Acceptance of Abstract for Presentation at ASCO Gastrointestinal Cancers Symposium 2025
IDENTIFYING THE OPPORTUNITY
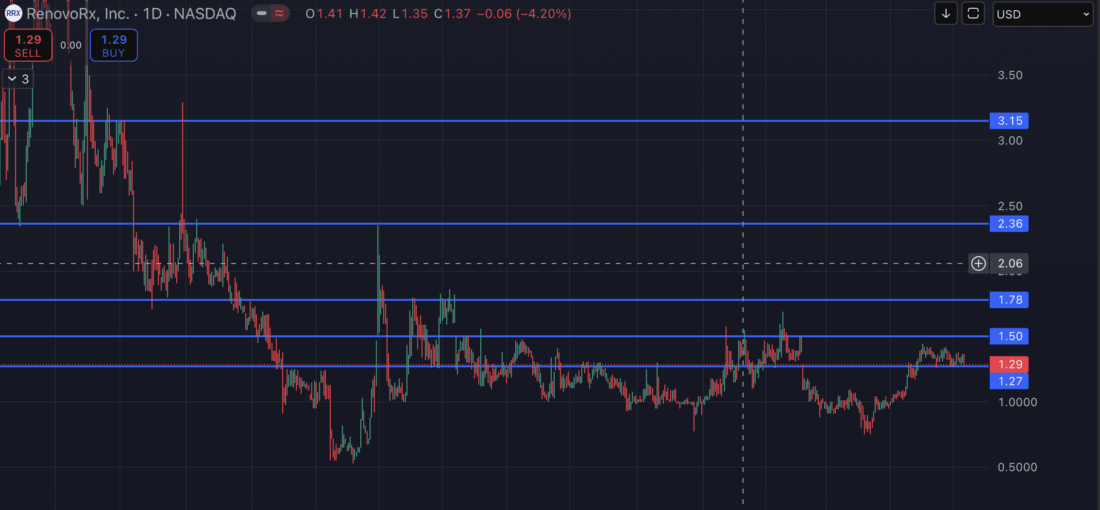

Price Action Is In A Major Area of Potential And Ready to Launch
This small float biotech locked and loaded!
Key Levels
Key Level #1: $1.50 (+17.19%)
Key Level #2: $1.78 (+39.06%)
Key Level #3: $2.36 (+84.38%)
Key Level #4: $3.15 (+146.09%)
Potential Support Key Level: $1.27
Innovative Treatment Platform
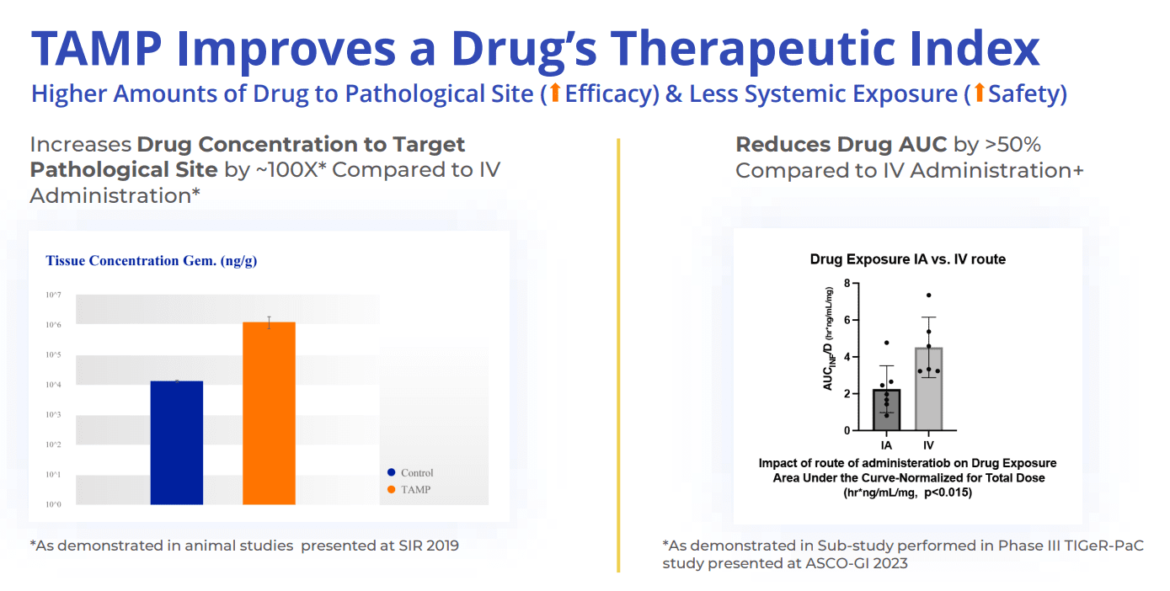
RenovoRx (NASDAQ: RNXT) is at the forefront of revolutionizing cancer treatment with its proprietary Trans-Arterial Micro-Perfusion (TAMP™) therapy platform. TAMP™ is an innovative approach designed to ensure the precise delivery of both existing and novel oncology agents directly to the tumor. This targeted method significantly minimizes the toxicities typically associated with systemic (intravenous) chemotherapy, offering patients increased safety, better tolerance, and improved efficacy. The lead product candidate utilizing this platform, RenovoGem™, is a novel drug-device combination currently undergoing evaluation in the Phase III TIGeR-PaC clinical trial for locally advanced pancreatic cancer (LAPC) under the oversight of the FDA’s Center for Drug Evaluation and Research.


The TAMP™ platform works by leveraging the peripheral vascular system for localized chemotherapy delivery. The patented delivery system, inserted through a small incision in the patient’s leg, features a double balloon design that allows physicians to isolate specific sections of the blood vessel. By adjusting the distance between the balloons, the system excludes branching blood vessels and creates the necessary pressure to push chemotherapy across the blood vessel wall, effectively bathing the tumor in the therapeutic agent. This localized approach is especially beneficial for tumors like those found in pancreatic cancer, which lack visible feeder blood vessels and are poorly served by traditional systemic chemotherapy.
The potential of the TAMP™ platform extends beyond pancreatic cancer. Its ability to deliver high concentrations of chemotherapy directly to the tumor site while reducing systemic exposure could transform the treatment landscape for various solid tumors. The ongoing Phase III TIGeR-PaC trial has already shown promising results, with a 6-month median overall survival benefit and a significant reduction in side effects compared to standard care. By continuing to refine and expand the application of the TAMP™ platform, RenovoRx aims to address critical gaps in cancer treatment, providing new hope for patients with difficult-to-treat tumors.

RenovoRX
(NASDAQ: RNXT)
Promising Clinical Pipeline
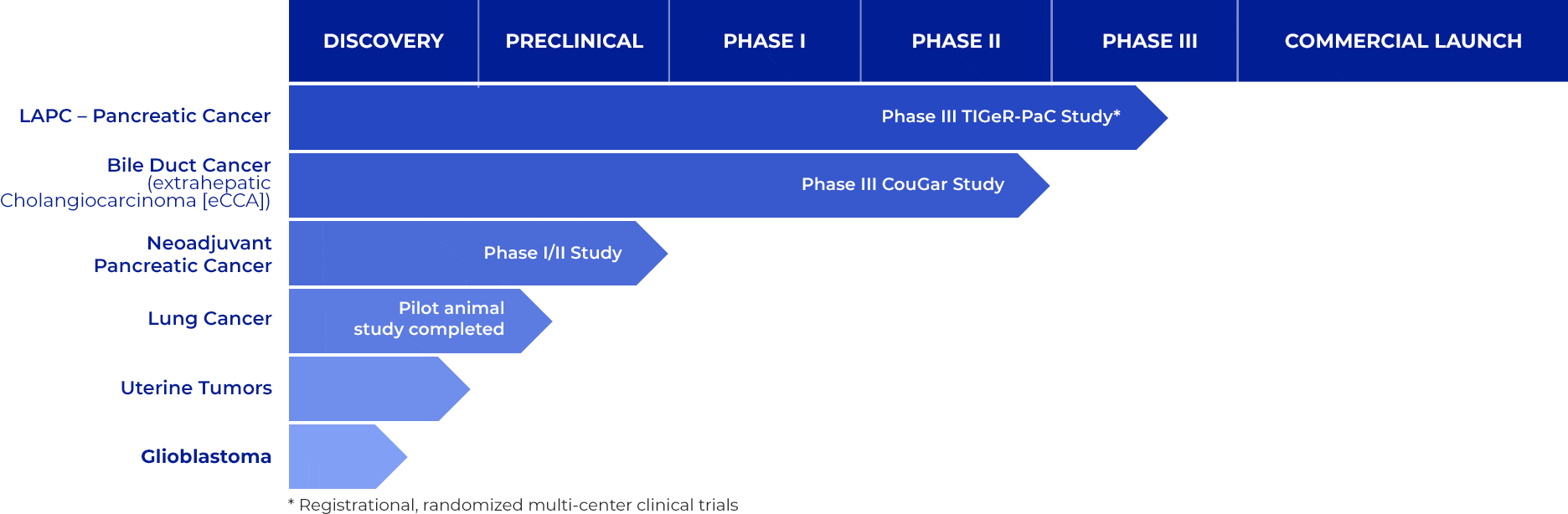

RenovoRx is dedicated to transforming the lives of cancer patients by developing innovative solutions that change the current paradigm of cancer care. The company's commitment is reflected in its lead product candidate, RenovoGem™, a novel oncology drug-device combination designed to deliver targeted chemotherapy with reduced systemic toxicity.
RenovoGem utilizes the proprietary Trans-Arterial Micro-Perfusion (TAMP™) therapy platform to provide localized delivery of gemcitabine, a chemotherapy agent, directly to tumor sites. This method aims to enhance the efficacy of the drug while minimizing the debilitating side effects commonly associated with systemic (intravenous) chemotherapy.
RenovoGem is currently being evaluated in the Phase III TIGeR-PaC clinical trial for the treatment of locally advanced pancreatic cancer (LAPC). This trial, regulated under the FDA’s 21 CFR 312 pathway, is investigating the effectiveness of RenovoGem in improving patient outcomes. The innovative approach of pressure-mediated delivery allows the chemotherapy to bathe the tumor tissue directly, potentially offering a significant improvement in treatment efficacy.
Additionally, RenovoGem has received FDA Orphan Drug Designation for pancreatic cancer, providing seven years of market exclusivity upon NDA approval. These designations and advancements in clinical trials underscore the potential for RenovoGem to become a groundbreaking treatment in the oncology field, attracting significant attention from investors seeking promising biopharmaceutical opportunities.
Beyond pancreatic cancer, RenovoGem is set to be evaluated in a Phase III clinical trial for bile duct cancer (extrahepatic cholangiocarcinoma, or eCCA) in late 2023. This rare and aggressive cancer forms in the bile ducts and is often diagnosed at an advanced stage, making effective treatment crucial.
RenovoRx has also secured FDA Orphan Drug Designation for cholangiocarcinoma, which could grant seven years of exclusivity if RenovoGem is approved for this indication. Further, RenovoRx is exploring preclinical studies to extend the application of RenovoGem to other solid tumors, including non-small cell lung cancer, uterine tumors, and glioblastoma, indicating a broad potential for its innovative therapy platform.
The expanding pipeline of RenovoGem applications represents a significant growth opportunity for RenovoRx, potentially driving substantial returns for investors.
The ability to target multiple high-need cancer indications with a single, versatile therapy platform enhances the company's market position and investment appeal. Additionally, the orphan drug designations and ongoing clinical trials signal strong regulatory support and a clear path to commercialization, making RenovoRx a compelling investment opportunity in the biopharmaceutical sector.
Strategic Market Position and Growth Potential
RenovoRx is strategically positioned to capture a significant share of the oncology market with its innovative therapy platform. By focusing on the targeted delivery of chemotherapy directly to tumors, RenovoRx aims to improve treatment efficacy and reduce harmful side effects, addressing a critical need in cancer care.
The global oncology market is expected to grow substantially over the next decade, driven by increasing cancer rates and advancements in treatments. RenovoRx’s TAMP™ platform is well-suited to capitalize on this growth, particularly with its potential applications for treating hard-to-reach tumors such as those in pancreatic and bile duct cancers. Pancreatic cancer alone has a worldwide incidence of 495,000 new cases annually, with 30% of these cases being locally advanced at presentation. In the U.S., it is projected to become the second leading cause of cancer-related deaths, accounting for 48,000 deaths annually.
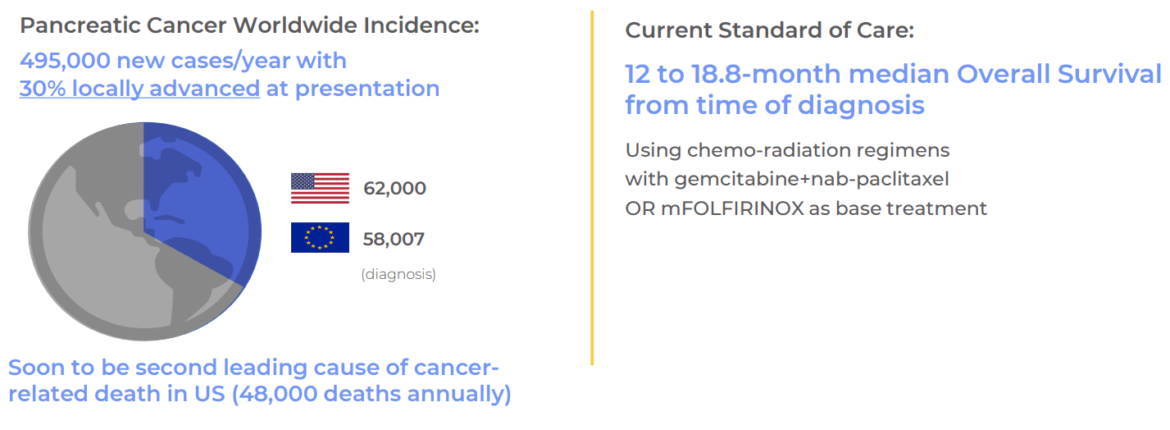

RenovoGem™, RenovoRx’s lead product candidate, is positioned to address the high unmet need in treating locally advanced pancreatic cancer (LAPC). The current standard of care for LAPC includes chemotherapy regimen, which offer a median overall survival of 12 to 18.8 months from diagnosis.
However, only three drugs have been approved by the FDA for LAPC in the past decade, each providing less than a two-month median overall survival benefit and associated with increased toxicity rates. RenovoGem aims to surpass these benchmarks, with independent interviews suggesting oncologists would likely adopt RenovoGem if it demonstrates a four-month overall survival benefit and improved toxicity profile.
Additionally, RenovoRx’s strong intellectual property and regulatory designations, such as FDA Orphan Drug status, provide valuable market exclusivity and protection. These factors enhance RenovoRx’s attractiveness as an investment, indicating substantial growth and potential returns for investors.
Experienced Leadership and Strategic Vision
RenovoRx boasts a seasoned management team with over 200 years of combined experience in drug development and commercialization. The team has a proven track record of successful FDA filings and blockbuster drug launches. Backed by a world-class board of directors and scientific advisors, RenovoRx is strategically positioned to advance its clinical pipeline, expand its innovative TAMP™ platform, and deliver significant value to investors.
Leadership Team


Shaun R. Bagai
Chief Executive Officer
Mr. Bagai has been the Chief Executive Officer and director of RenovoRx since June 2014. Prior to this role, he led Global Market Development at HeartFlow, Inc. from 2011 to 2014, where he directed Japanese market research, regulatory and payer collaboration, and Key Opinion Leader development. At HeartFlow, he orchestrated their largest clinical trial and secured the company's first global customers. Mr. Bagai has a proven track record of launching innovative technologies in both large corporations and growth-phase companies, including developing the European market for renal denervation, which led to Medtronic’s acquisition of Ardian, Inc. in 2011. He holds a BSc. in Biology/Pre-Med from the University of California, Santa Barbara.


Ramtin Agah, MD
Chief Medical Officer, Founder
Dr. Agah has served as Chief Medical Officer and Co-Founder of RenovoRx since December 2009, and as Chairman of the Board since May 2018. He is currently an Interventional Cardiologist at El Camino Hospital, Mountain View, a position he has held since September 2005. Since July 2012, he has also been a physician consultant for Abbott Vascular. Previously, Dr. Agah was an Assistant Professor of Internal Medicine in the Division of Cardiology at the University of Utah. He completed a Fellowship in Interventional Cardiology at the Cleveland Clinic Foundation, a Residency in Internal Medicine at Baylor College of Medicine, and a Fellowship in Cardiology at the University of California, San Francisco. Dr. Agah earned his MD from the University of Texas Southwestern Medical School.


Leesa Gentry
Chief Clinical Officer
Ms. Gentry brings 29 years of experience in improving clinical research programs within the Contract Research Organization (CRO), pharmaceutical, and biotech industries. She has held key positions at IQVIA (Quintiles), PPD, OmnicareCR, and Otsuka. Prior to joining RenovoRx, she was Senior Vice President of Clinical Operations at Evotec (NASDAQ: EVO). At PPD, she served as Global Project Manager and Senior Research Specialist for Clinical Systems, where her team developed one of the pharmaceutical industry’s first clinical trial management systems. At Omnicare, she expanded the clinical research site network within long-term care, with her work being featured in the Good Clinical Practice Journal. During her tenure at Otsuka, she developed novel methods for improved trial implementation in clinical operations, data management, and third-party collaboration, with her work on registration trial implementation in resource-constrained environments being featured in the Bulletin of the World Health Organization. Ms. Gentry holds a B.A. in Psychology and an M.S. in Gerontology from the University of Central Missouri, and an M.A. in Psychology from the University of Mary Hardin-Baylor.
Scientific Advisory Board


Mike Pishvaian, MD, PhD
Associate Professor, Department of Oncology Director of the Gastrointestinal, Developmental Therapeutics, and Clinical Research Programs at the NCR Kimmel Cancer Center at Sibley Memorial Hospital
Johns Hopkins University School of Medicine


Karyn A. Goodman, MD, MS
Professor and Vice Chair of Clinical Research, Department of Radiation Oncology, Icahn School of Medicine at Mount Sinai
Associate Director of Clinical Research, The Tisch Cancer Institute at Mount Sinai


Margaret A. Tempero, M.D.
Professor of Medicine and Director of the UCSF Pancreas Center
Editor-in-Chief of JNCCN
Former ASCO President


Michel Ducreux, M.D., Ph.D.
Head of the Gastrointestinal Oncology Unit and Gastrointestinal Oncology Tumor Board at Gustave Roussy
Professor of Oncology at Paris-Saclay University in France
Vice-Chair of ESMO GI
Sources
- Source 1: https://43577226.fs1.hubspotusercontent-na1.net/hubfs/43577226/RenovoRX%20RNXT%20info%20for%20investors%20May%202024.pdf
- Source 2: https://renovorx.com/about-us/leadership-team/
- Source 3: https://renovorx.com/
- Source 4: https://renovorx.com/about-us/
- Source 5: https://renovorx.com/about-us/advisory-board/
- Source 6: https://renovorx.com/clinical-trials/about-tiger-pac/
- Source 7: https://renovorx.com/tamp/
- Source 8: https://renovorx.com/pipeline-overview/
- Source 9: https://finance.yahoo.com/news/publication-results-pre-clinical-studies-130000365.html
- Source 10: https://finance.yahoo.com/news/renovorx-ceo-shaun-bagai-present-123000434.html
- Source 11: https://finance.yahoo.com/news/renovorx-increases-production-fda-cleared-123000871.html
- Source 12: https://finance.yahoo.com/news/renovorx-announces-acceptance-abstract-presentation-130000749.html
Disclaimer
Disclaimer
Stock Research Today is a project of Virtus Media Group LLC and intended solely for entertainment and informational purposes. This website / media webpage is owned, operated and edited by Virtus Media Group LLC. Any wording found on this website / media webpage or disclaimer referencing “I” or “we” or “our” or “Virtus Media” refers to Virtus Media Group LLC. This website / media webpage is a paid advertisement, not a recommendation nor an offer to buy or sell securities. By reading our website / media webpage you agree to the terms of this disclaimer, which are subject to change at any time. We are not registered or licensed in any jurisdiction whatsoever to provide investment or brokerage advice or anything of an advisory or consultancy nature and therefore are unqualified to give investment recommendations. Always do your own research and consult with a licensed investment professional before investing. This communication is never to be used as the basis for making investment decisions and is for entertainment and educational purposes only. At most, this communication should serve as a starting point to do your own research and consult with a licensed professional regarding the companies profiled and discussed. Companies with low price per share are speculative and carry a high degree of risk, so only invest what you can afford to lose. Consult your financial, investment and tax advisors to determine what financial and tax strategies may be right for you. Investor protection and other important information is available at https://www.sec.gov/.
By using our service, you agree not to hold our site, its editors, owners, or staff liable for any damages, financial or otherwise, that may occur due to any action you may take based on the information contained within or referred to from our website / media webpage. We do not advise any reader take any specific action. Losses can be larger than expected if the company experiences any problems with liquidity or wide spreads. Never invest purely based on our alerts. Gains mentioned in our website / media webpage may be based on end-of-day or intraday data.
This publication and its owners and affiliates may hold positions in the securities mentioned in our alerts, which we may sell at any time without notice to our subscribers, which may have a negative impact on share prices. If we own any shares we will list the information relevant to the stock and number of shares here. That information is only valid at the time it is published, and we do not undertake to update it.
Virtus Media’s business model is to receive financial compensation to promote public companies and to conduct investor relations advertising, marketing and publicly disseminate information, not limited to our websites, email, sms, push notifications, influencers, social media postings, ticker tags, press releases, online interviews, podcasts, videos, audio ads, banner ads, native ads, and responsive ads. This compensation is a major conflict of interest in our ability to be unbiased regarding the subject of our reports and communications. Therefore, this communication should be viewed as a commercial advertisement only. We have not investigated the background of the parties who hired us, or of the profiled companies. Any non-compensated alerts are purely for the purpose of expanding our database for the benefit of our future financially compensated investor relations efforts.
The third parties paying for our services, the profiled company, or their affiliates likely wish to liquidate shares of the profiled company at or near the time you receive this communication, which has the potential to impact share prices, possibly significantly. Frequently companies profiled in our alerts experience a large increase in volume and share price during the course of investor relations marketing, which may end as soon as the investor relations marketing ceases.
We do not guarantee the timeliness, accuracy, or completeness of the information on our website / media webpage. The information in our website / media webpage is believed to be accurate and correct, but has not been independently verified and is not guaranteed to be correct. The information is collected from public sources, such as the profiled company’s website and press releases, but is not researched or verified in any way whatsoever to ensure the publicly available information is correct. Furthermore, Virtus Media often employs independent contractor writers who may make errors when researching information and preparing these communications regarding profiled companies. Independent writers’ works are double-checked and verified before publication, but it is possible for errors or omissions to take place during editing of independent contractor writer’s communications regarding the profiled company(s). You should assume all information in all of our communications is incorrect until you personally verify the information, and again are encouraged to never invest based on the information contained in our written communications. Our emails may contain forward-looking statements, which are not guaranteed to materialize due to a variety of factors. Compensation: Pursuant to an agreement between Virtus Media Group LLC and JRZ Capital LLC, Virtus Media Group LLC has been hired by JRZ Capital LLC for a period beginning on 05/28/2024 and ending 06/28/2024 to publicly disseminate information about NASDAQ:RNXT via digital communications. We have been paid fifty thousand dollars USD. Pursuant to a further agreement between Virtus Media Group LLC and JRZ Capital LLC, Virtus Media Group LLC has been hired by JRZ Capital LLC for a period beginning on 09/23/2024 and ending on 10/11/2024 to publicly disseminate information about NASDAQ:RNXT via digital communications. We have been paid forty five thousand dollars USD. Pursuant to a further agreement between Virtus Media Group LLC and JRZ Capital LLC, Virtus Media Group LLC has been hired by JRZ Capital LLC for a period beginning on 11/07/2024 and ending on 11/28/2024 to publicly disseminate information about NASDAQ:RNXT via digital communications. We have been paid fifty thousand dollars USD. Virtus Media Group has been hired by JRZ Capital LLC for a period beginning on 07/16/2025 and ending 08/15/2025 to publicly disseminate information about NASDAQ:RNXT via digital communications. We have been paid sixty five thousand dollars USD. Social Media Influencer Compensation: Virtus Media Group LLC agrees to pay social media influencer eight thousand dollars and social media influencer three hundred fifty dollars and social media influencer one hundred fifty dollars and social media influencer one thousand five hundred dollars and The Investing Authority LLC twelve thousand dollars. Virtus Media Group LLC agrees to pay The Investing Authority fourteen thousand five hundred USD. Virtus Media Group LLC agrees to pay Social Media Influencer six thousand five hundred USD. Virtus Media Group LLC agrees to pay Social Media Influencer five thousand USD. Virtus Media Group LLC agrees to pay social media influencer four thousand dollars and social media influencer one thousand five hundred dollars and social media influencer five hundred dollars and social media influencer one thousand dollars. Virtus Media Group LLC agrees to pay The Investing Authority ten thousand dollars USD and social media influencer #1 two thousand dollars USD and social media influencer two thousand five hundred dollars USD and social media influencer #3 six hundred ninety dollars USD and social media influencer sixty dollars USD. Virtus Media Group LLC agrees to pay social media influencer six thousand five hundred USD.

