LATEST NEWS
OS Therapies Announces Pricing of its Initial Public Offering on NYSE American - Company to Trade under Symbol “OSTX”
5 Reasons Why OS Therapies Inc (NYSE: OSTX) Could Be Poised For Significant Upside Potential in 2024
IDENTIFYING THE OPPORTUNITY

OS Therapeutics Inc
(NYSE: OSTX)
Addressing Unmet Medical Need in Kids and Adults


OS Therapeutics Inc (NYSE: OSTX) is a clinical stage therapeutic company focused on the identification, development and commercialization of treatments for Osteosarcoma (OS) and other solid tumors. OS Therapies was launched to meet significant unmet need for new treatments in cancers of the bone in kids and adults.
Osteosarcoma is an extremely challenging and often aggressive cancer that has particular treatment challenges due to location, changing genotypes, and high recurrence rates. This company's mission is to answer the calls for new treatments, considering there have been no new treatments for OS in over 30 years.
OS Therapeutics Inc (NYSE: OSTX) has expanded the pipeline beyond Osteosarcoma with OST-HER2 into other solid tumors with the same recurrence mechanism of action, including Breast, Esophageal, and Lung cancer. With the addition of OST-tADC, considered a next generation Antibody Drug Conjugate (ADC) platform technology, they will be widening their scope to Ovarian, Lung, and Pancreatic cancer.
The goal of the company is to identify lead candidates in the treatment of Osteosarcoma and other solid tumors for clinical development, regulatory approval and commercialization. Starting with the most common genetic mutation found in Osteosarcoma, OS Therapies has identified a lead candidate in HER-2 Osteosarcoma with a goal of rapid clinical and regulatory analysis and review. This will be immediately follows in parallel with the OST-tADC development.
Company Timeline
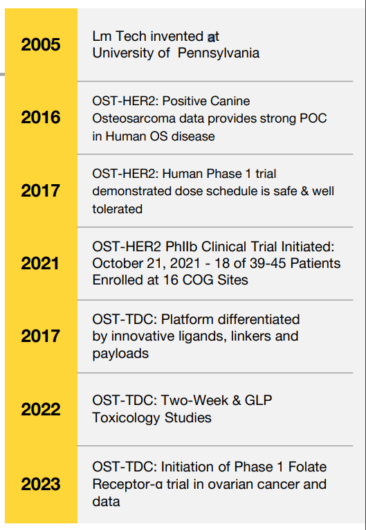

The Details: OST-HER2 Mechanism of Action (MOA)
The intravenous OST-HER2 vector is rapidly cleared by the immune system’s antigen-presenting cells (APCs). Once inside the body, these APCs are strongly activated and generate potent HER2-specific T cells from within the patient. These T cells then proliferate and travel through the bloodstream, where they are attracted to and hunt down micro-metastases. As they target these cancerous cells, their contents spill out, revealing additional cancer targets to the immune system.
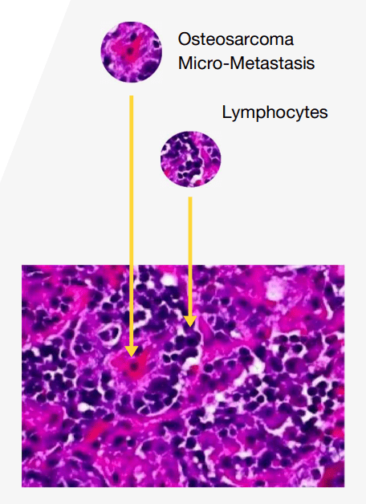

The exposure of new cancer targets leads to the generation of further T cells specifically targeting these newly identified antigens. This ongoing process ensures that the immune response continues to adapt and expand, effectively extending the treatment's reach and duration. Through this cycle, the OST-HER2 vector enhances the immune system's ability to combat cancer by continually revealing and targeting new cancerous cells.
OS Therapies Inc
(NYSE: OSTX)
A Robust Pipeline of Innovative Therapies
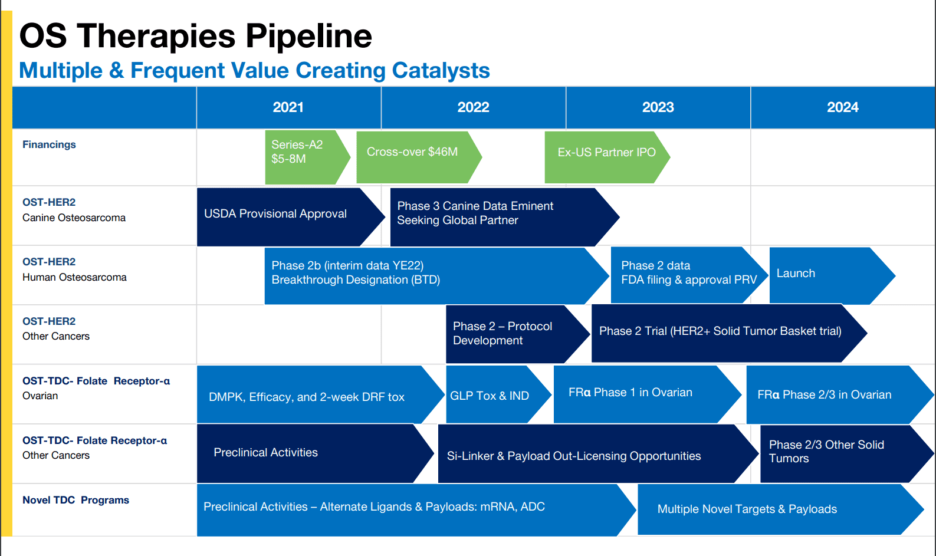

OS Therapies stands out in the biotechnology sector with its robust pipeline of innovative therapies targeting cancer and other high-impact diseases. The company's lead drug candidate, OSTE-001, is currently in advanced clinical trials, demonstrating promising efficacy and safety profiles. This candidate represents a significant leap forward in cancer treatment, offering a potential breakthrough for patients with limited options. The diverse range of therapies in development also underscores the company's commitment to addressing unmet medical needs and expanding its market potential.
The pipeline includes several preclinical and early-stage candidates, each designed to tackle different aspects of cancer biology. These candidates leverage cutting-edge technologies, including gene editing and novel drug delivery systems, positioning OS Therapies at the forefront of biotechnology innovation. The breadth and depth of the pipeline not only highlight the company's scientific prowess but also its strategic approach to diversifying risk and maximizing opportunities for success.
Furthermore, OS Therapies' dedication to advancing its pipeline is evident through its strategic partnerships and collaborations with leading research institutions and industry players. These alliances enhance the company's research capabilities and accelerate the development of its therapies. The collaborative approach helps in validating the therapeutic potential of their candidates and provides access to additional resources and expertise.
The Link Between Human & Canine Osteosarcoma
The company’s canine trials are a significant step forward in ensuring the safety and efficacy of their treatments before advancing to human trials. By conducting these trials in dogs, researchers can closely monitor the effects of the OST-HER2 vector in a model that shares similar physiological and immunological characteristics with humans. Canines, particularly those with naturally occurring cancers, provide a valuable opportunity to assess how the treatment performs in a real-world, biological setting. This can help identify potential side effects, determine appropriate dosage levels, and refine the treatment protocol based on empirical data from these trials.
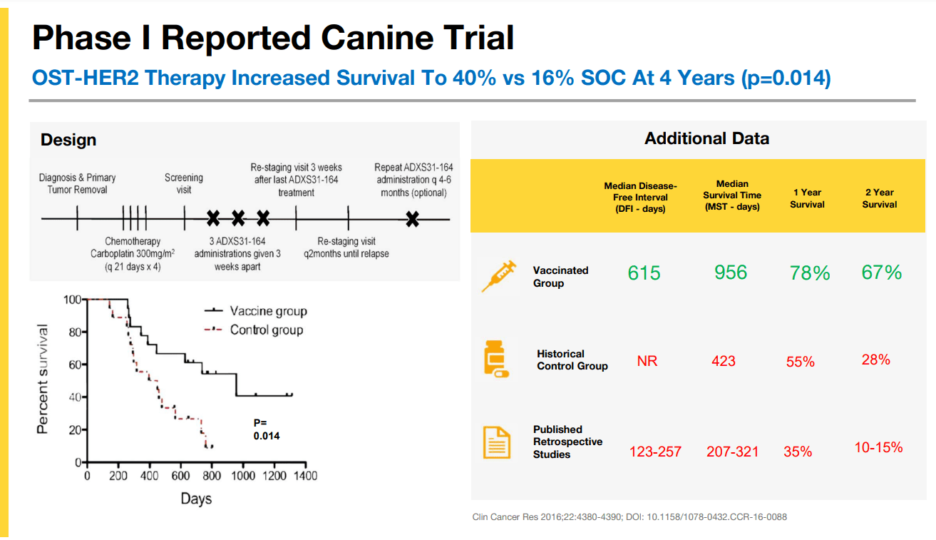

The independent NIH Phase III canine trial (COTC 026) evaluating the OST-HER2 treatment arm has yielded promising preliminary results, with a p-value of 0.007 indicating statistically significant benefits. This trial, designed as a multi-arm study, includes several treatment arms compared against the standard of care (SoC) for canine osteosarcoma. The SoC consists of surgery followed by four doses of carboplatin, while the OST-HER2 arm involves SoC followed by three doses of the OST32-64 vector. Other arms in the study test various treatments, such as rapamycin, oncolytic VSV, and inhaled rHu IL-15. The primary endpoint is the disease-free interval (DFI), with secondary endpoints assessing overall survival compared to SoC.
Preliminary data from the Kaplan-Meier survival curves show that the OST-HER2 treatment arm demonstrates a threefold improvement in both overall survival and DFI compared to the SoC. Notably, the OST-HER2 curve is less mature than the SoC curve, which has more data accumulated over time. The OST-HER2 arm exhibits significant curve separation and the preliminary Gehan-Breslow-Wilcoxon test p-value of 0.007 supports early separation in survival curves. This early indication of efficacy suggests that as the study progresses and more events occur, the benefits of the OST-HER2 treatment may become even more pronounced. Experts anticipate that the final topline data, expected within the first half of 2022, will likely reveal even more substantial improvements in the OST-HER2 arm as the data matures.
Human Trials
The company's human trials for the OST-HER2 treatment have generated significant interest due to their promising preliminary outcomes. These trials are designed to evaluate the safety and efficacy of the OST-HER2 vector in patients with HER2-positive cancers. The initial phases have shown encouraging results, with the therapy demonstrating a good safety profile and early indications of effectiveness. The OST-HER2 treatment has been well-tolerated by participants, with manageable side effects and no severe adverse events reported. These findings are crucial for building confidence in the therapy's potential for broader application in treating HER2-positive cancers.
Furthermore, the human trials have provided valuable insights into the therapy's mechanism of action and its impact on cancer progression. Preliminary data suggest that the OST-HER2 treatment can generate a robust immune response against HER2-positive cancer cells, leading to improved disease control and potentially enhanced survival rates. The ongoing trials continue to monitor the long-term effects and overall survival benefits, with hopes that the positive trends observed so far will translate into substantial clinical benefits. As the trials advance, they will play a critical role in determining the therapy's future in oncology and its potential for regulatory approval and widespread use.
OS Therapeutics Inc
(NYSE: OSTX)
Strong Financial Position and Funding History

OS Therapies' strong financial position and impressive funding history make it an attractive investment opportunity. The company has successfully raised substantial capital through multiple funding rounds, including a recent Series C financing round that significantly bolstered its financial reserves. This robust funding history reflects investor confidence in the company's vision and its ability to deliver on its strategic objectives.
The company’s sound financial management is evident from its strategic allocation of funds towards critical areas such as research and development, clinical trials, and operational expansion. OS Therapies has demonstrated prudent financial stewardship, ensuring that its resources are efficiently used to advance its therapeutic pipeline and achieve key milestones. This effective financial strategy enhances the company's ability to sustain long-term growth and achieve its objectives.
Moreover, the company's strong investor base includes notable venture capital firms and strategic partners, which provides a solid foundation for future fundraising and collaboration opportunities. This network not only supports the company's financial stability but also opens doors to potential strategic alliances and market expansion. The continued support from a diverse group of investors further validates the company's potential and strategic direction.
Interview Highlights from Key Opinion Leader Experts


Introducing the Management Team of OS Therapies Inc.
(NYSE: OSTX)


Paul Romness, MHPChair, CEO - OS Therapies
Mr. Paul Romness leads OS Therapeutics with over 25 years of experience in the biopharmaceutical industry having served every function within major companies like Johnson & Johnson, Amgen and Boehringer Ingelheim. He has been directly involved in the launch of 9 major products in the industry covering indications for oncology, surgery, HIV, FSD, COPD, IPF, cardiovascular and diabetes. Throughout his professional career and within his community he has focused on and advocated for unmet medical need and getting treatments to patients. Mr. Romness has a B.S. in Finance from American University and a Masters of Health Policy from George Washington University Medical Center.


Robert Petit, PhD | Chief Medical & Scientific Officer Founding Scientist: OST-HER2
Dr. Robert Petit is an accomplished biopharma executive, innovator/inventor, company builder, and medical scientist. His personal mission is to develop new products and treatments that improve and extend the lives of patients. Robert has C-Suite experience leading several public and private therapeutic companies in the biotechnology, oncology, immunology, and infectious disease spaces. He has a consistent track record of excellence in corporate strategy, clinical development, scientific development, pipeline determination, medical and regulatory affairs.


Gerald Commissiong | Chief Business Officer
Mr. Commissiong is healthcare executive with over 15 years of experience serving in C-suite roles in emerging growth companies developing and commercializing novel therapeutics, diagnostics and natural products to address acute and chronic diseases. He previously served as President & CEO of Amarantus Bioscience Holdings, Inc. and Todos Medical, Ltd. helping raise over $70 million in equity and debt capital. His experience spans neurology, regenerative medicine, oncology and infectious disease. Mr. Commissiong received a BS in Management Science & Engineering with a focus on financial decisions from Stanford University and played professional football in the Canadian Football League for the Calgary Stampeders.


Jutta Wanner, PhD | Advisor Founding Scientist: OST-tADC
Dr. Jutta Wanner joins her Tunable Drug Conjugate (Advanced ADC) technology from BlinkBio, where she was Chief Scientific Officer. Before that she was at Roche where she was a co-lead in discovery chemistry. Dr. Wanner brings expertise across multiple therapeutic areas including oncology, inflammation and virology. Dr. Wanner received her PhD from the University of Kansas and conducted her postdoctoral training at The Scripps Research Institute in San Diego.


Jack Doll | Chief of Staff
Mr. Jack Doll is a Research Rock Star and scanning/transmission electron microscope guru. His final research dissertation was on Drug/Antibody-loaded Nanoparticles for Cancer Treatment. A recent Biomaterials and Engineering graduate from the University of Georgia, Jack assists Dr. Petit on the Osteosarcoma Phase IIb Clinical Trial, as well as the final toxicology trial in Tunable Drug Conjugates.
Sources
- Source 1: https://ostherapies.com/leadership/
- Source 2: https://ostherapies.com/
- Source 3: https://ostherapies.com/technology/
- Source 4: https://finance.yahoo.com/quote/OSTX/
- Source 5: https://ostherapies.com/trials/
- Source 6: https://ir.ostherapies.com/
- Source 7: https://ir.ostherapies.com/news-events/press-releases/detail/20/os-therapies-forms-osteosarcoma-patient-advocacy-advisory
- Source 8: https://ir.ostherapies.com/company-information
- Source 9: https://ir.ostherapies.com/stock-data/quote
- Source 10: https://d1io3yog0oux5.cloudfront.net/_b76ceebcbe0396766471db74436f8c69/ostherapies/db/2484/23266/pdf/OST_Summer_2022.pdf
Disclaimer
Disclaimer
Stock Research Today is a project of Virtus Media Group LLC and intended solely for entertainment and informational purposes. This website / media webpage is owned, operated and edited by Virtus Media LLC. Any wording found on this website / media webpage or disclaimer referencing “I” or “we” or “our” or “Virtus Media” refers to Virtus Media LLC. This website / media webpage is a paid advertisement, not a recommendation nor an offer to buy or sell securities. By reading our website / media webpage you agree to the terms of this disclaimer, which are subject to change at any time. We are not registered or licensed in any jurisdiction whatsoever to provide investment or brokerage advice or anything of an advisory or consultancy nature and therefore are unqualified to give investment recommendations. Always do your own research and consult with a licensed investment professional before investing. This communication is never to be used as the basis for making investment decisions and is for entertainment and educational purposes only. At most, this communication should serve as a starting point to do your own research and consult with a licensed professional regarding the companies profiled and discussed. Companies with low price per share are speculative and carry a high degree of risk, so only invest what you can afford to lose. Consult your financial, investment and tax advisors to determine what financial and tax strategies may be right for you. Investor protection and other important information is available at https://www.sec.gov/.
By using our service, you agree not to hold our site, its editors, owners, or staff liable for any damages, financial or otherwise, that may occur due to any action you may take based on the information contained within or referred to from our website / media webpage. We do not advise any reader take any specific action. Losses can be larger than expected if the company experiences any problems with liquidity or wide spreads. Never invest purely based on our alerts. Gains mentioned in our website / media webpage may be based on end-of-day or intraday data.
This publication and its owners and affiliates may hold positions in the securities mentioned in our alerts, which we may sell at any time without notice to our subscribers, which may have a negative impact on share prices. If we own any shares we will list the information relevant to the stock and number of shares here. That information is only valid at the time it is published, and we do not undertake to update it.
Virtus Media’s business model is to receive financial compensation to promote public companies and to conduct investor relations advertising, marketing and publicly disseminate information, not limited to our websites, email, sms, push notifications, influencers, social media postings, ticker tags, press releases, online interviews, podcasts, videos, audio ads, banner ads, native ads, and responsive ads. This compensation is a major conflict of interest in our ability to be unbiased regarding the subject of our reports and communications. Therefore, this communication should be viewed as a commercial advertisement only. We have not investigated the background of the parties who hired us, or of the profiled companies. Any non-compensated alerts are purely for the purpose of expanding our database for the benefit of our future financially compensated investor relations efforts.
The third parties paying for our services, the profiled company, or their affiliates likely wish to liquidate shares of the profiled company at or near the time you receive this communication, which has the potential to impact share prices, possibly significantly. Frequently companies profiled in our alerts experience a large increase in volume and share price during the course of investor relations marketing, which may end as soon as the investor relations marketing ceases.
We do not guarantee the timeliness, accuracy, or completeness of the information on our website / media webpage. The information in our website / media webpage is believed to be accurate and correct, but has not been independently verified and is not guaranteed to be correct. The information is collected from public sources, such as the profiled company’s website and press releases, but is not researched or verified in any way whatsoever to ensure the publicly available information is correct. Furthermore, Virtus Media often employs independent contractor writers who may make errors when researching information and preparing these communications regarding profiled companies. Independent writers’ works are double-checked and verified before publication, but it is possible for errors or omissions to take place during editing of independent contractor writer’s communications regarding the profiled company(s). You should assume all information in all of our communications is incorrect until you personally verify the information, and again are encouraged to never invest based on the information contained in our written communications. Our emails may contain forward-looking statements, which are not guaranteed to materialize due to a variety of factors.
Compensation: Pursuant to an agreement between Virtus Media LLC and The Investing Authority LLC, The Investing Authority (co-owned by a member of Virtus Media Group LLC) hired Virtus Media Group LLC for a period beginning on 2024-08-07 and ending 2024-09-17 to publicly disseminate information about NYSE: OSTX via digital communications. We have been paid thirty five thousand dollars USD. Pursuant to an ag reement between Virtus Media LLC and The Investing Authority LLC, The Investing Authority (co-owned by a member of Virtus Media Group LLC) hired Virtus Media Group LLC for a period beginning on 7/4/2025 and ending after one day to publicly disseminate information about NYSE: OSTX via digital communications. We have been paid fifteen thousand dollars USD.

