LATEST NEWS
Vistagen enrolls first subject in Phase III trial of social anxiety disorder drug
IDENTIFYING THE OPPORTUNITY
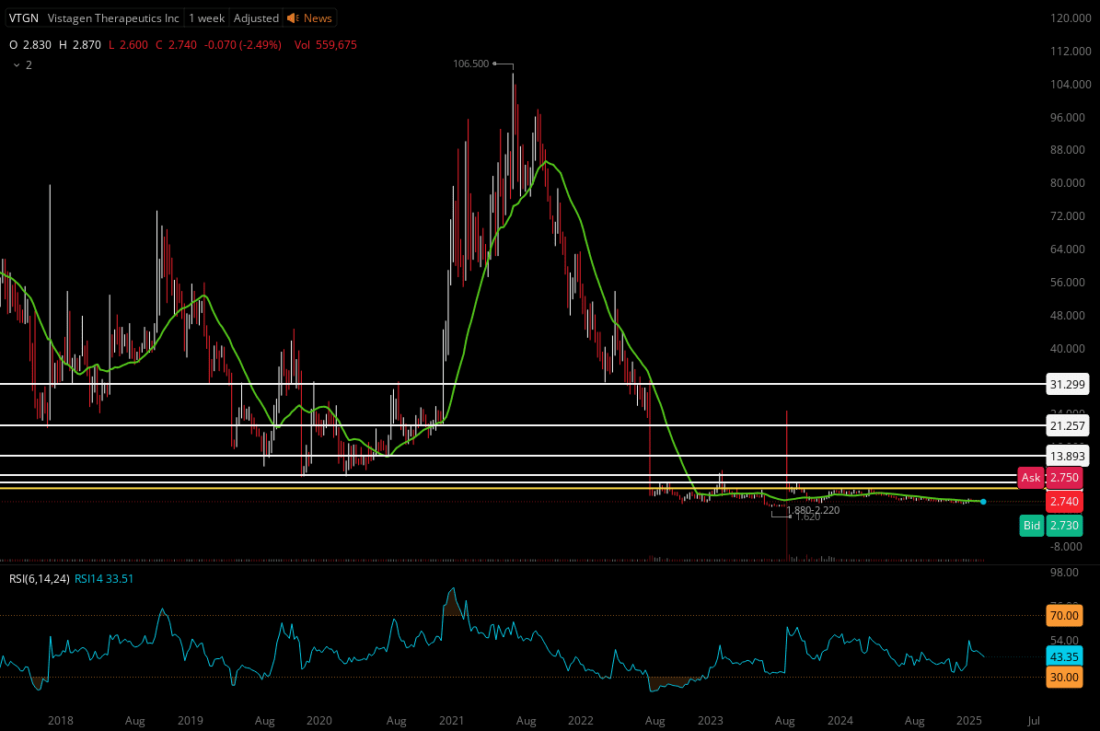

INSTITUTIONAL SUPPORT IS IN PLACE AND READY TO RUN
We are in a significant blast zone and are seeing sparks
TARGETS
Key Level #1: $6.00 (+118.98%)
Key Level #2: $7.42 (+170.80%)
Key Level #3: $9.21 (+236.13%)
Key Level #4: $13.89 (+406.93%)
Key Level #5: $22.59 (+724.45%)
Potential Support: $1.48
vISTAGEN
(NSDQ: vtgn)
The Science Behind Pherine-Based Therapies


Unlike traditional antidepressants and anxiety medications, Vistagen’s pherine-based therapies work by activating nasal chemosensory receptors, triggering immediate neurological responses. This mechanism enables:
- Fast-acting relief – Effects are felt within minutes of administration.
- No systemic exposure – Reducing risks of sedation, addiction, or cognitive impairment.
- User-friendly nasal spray format – Improving patient compliance and accessibility.
This unique approach positions Vistagen at the forefront of next-generation mental health treatments.
Fasedienol: A Potential Game-Changer for Social Anxiety Disorder (SAD)
Social Anxiety Disorder affects over 10% of the U.S. population, yet no new FDA-approved treatments have emerged in more than 20 years. Traditional medications like SSRIs and benzodiazepines are associated with slow onset times and undesirable side effects.
Vistagen’s Fasedienol Nasal Spray (PH94B) offers:
- Rapid relief of anxiety symptoms without sedation or cognitive impairment.
- No risk of dependency – making it a safer alternative to benzodiazepines.
- Potential for long-term use with favorable safety data.
Recent milestones:
- Positive Phase 2A trial results demonstrating fasedienol’s ability to reduce social anxiety symptoms within minutes.
- Initiation of repeat-dose study evaluating long-term safety and efficacy.
- Regulatory progress that paves the way for potential FDA approval.
VISTAGEN
(NSDQ: VTGN)
Building a Stronger Market Position
Vistagen has been strategically enhancing its market position through intellectual property development and clinical advancements. The recently granted U.S. patent for AV-101 bolsters the company’s ability to commercialize its treatments in the competitive mental health landscape.
Additionally, the company’s collaborations and licensing opportunities provide further validation for its technologies, setting the stage for long-term market adoption.


Mental health treatment is in desperate need of innovation, and Vistagen’s therapies could fill a critical gap in psychiatric care. With strong clinical data, strategic intellectual property, and a growing pipeline, Vistagen is advancing towards key regulatory milestones that could reshape the mental health space.
Vistagen
(NSDQ: VTGN)
Vistagen's Leadership: Experienced and Committed
Maurizio Fava, M.D.
Harvard University - Chairman
Professor of Psychiatry, Harvard Medical School; Director, Division of Clinical Research, Massachusetts General Hospital (MGH) Research Institute; and Executive Vice Chair of the Department of Psychiatry
Thomas Laughren, M.D.
Division Director for the Division of Psychiatry Products, U.S. FDA (retired)
Director (retired), U.S. Food and Drug Administration (FDA) Division of Psychiatry Products, Office of New Drugs, Center for Drug Evaluation and Research (CDER)
Michael Liebowitz, M.D.
Columbia University; The Medical Research Network
Former Columbia University psychiatrist, director and founder of the Anxiety Disorders Clinic at the New York State Psychiatric Institute; current Managing Director of The Medical Research Network LLC
Sanjay Mathew, M.D.
Baylor College of Medicine
Vice Chair for Research and Professor of Psychiatry and Behavioral Sciences at Baylor College of Medicine; Staff Psychiatrist at the Michael E. DeBakey VA Medical Center
Gerard Sanacora, Ph.D., M.D.
Yale University
Professor of Psychiatry, YaleSchool of Medicine; Director, Yale Depression Research Program; Co-Director, Yale-New Haven Hospital Interventional Psychiatry Service
Mark Wallace, M.D.
University of California San Diego
Professor of Clinical Anesthesiology, Chair of the Division ofPain Medicine, Medical Director and Director at the University of California, San Diego
Sources
- Source 1: https://www.vistagen.com/
- Source 2: https://www.vistagen.com/pipeline/overview
- Source 3: https://www.vistagen.com/investors/overview
- Source 4: https://www.vistagen.com/static-files/b1bcf087-576a-4e58-bd6c-4f5cdd9ad907
- Source 5: https://www.vistagen.com/news-releases/news-release-details/vistagen-initiates-palisade-3-phase-3-study-fasedienol-acute
- Source 6: https://www.vistagen.com/news/press-releases
- Source 7: https://www.vistagen.com/investors/overview
- Source 8: https://www.vistagen.com/about/advisory-board
- Source 9: https://www.vistagen.com/about/advisory-board
- Source 10: https://www.vistagen.com/presentations
- Source 11: https://finance.yahoo.com/news/vistagen-enrols-first-subject-phase-085224393.html
Disclaimer
Disclaimer
Stock Research Today is a project of Virtus Media Group LLC and intended solely for entertainment and informational purposes. This website / media webpage is owned, operated and edited by Virtus Media LLC. Any wording found on this website / media webpage or disclaimer referencing “I” or “we” or “our” or “Virtus Media” refers to Virtus Media LLC. This website / media webpage is a paid advertisement, not a recommendation nor an offer to buy or sell securities. By reading our website / media webpage you agree to the terms of this disclaimer, which are subject to change at any time. We are not registered or licensed in any jurisdiction whatsoever to provide investment or brokerage advice or anything of an advisory or consultancy nature and therefore are unqualified to give investment recommendations. Always do your own research and consult with a licensed investment professional before investing. This communication is never to be used as the basis for making investment decisions and is for entertainment and educational purposes only. At most, this communication should serve as a starting point to do your own research and consult with a licensed professional regarding the companies profiled and discussed. Companies with low price per share are speculative and carry a high degree of risk, so only invest what you can afford to lose. Consult your financial, investment and tax advisors to determine what financial and tax strategies may be right for you. Investor protection and other important information is available at https://www.sec.gov/.
By using our service, you agree not to hold our site, its editors, owners, or staff liable for any damages, financial or otherwise, that may occur due to any action you may take based on the information contained within or referred to from our website / media webpage. We do not advise any reader take any specific action. Losses can be larger than expected if the company experiences any problems with liquidity or wide spreads. Never invest purely based on our alerts. Gains mentioned in our website / media webpage may be based on end-of-day or intraday data.
This publication and its owners and affiliates may hold positions in the securities mentioned in our alerts, which we may sell at any time without notice to our subscribers, which may have a negative impact on share prices. If we own any shares we will list the information relevant to the stock and number of shares here. That information is only valid at the time it is published, and we do not undertake to update it.
Virtus Media’s business model is to receive financial compensation to promote public companies and to conduct investor relations advertising, marketing and publicly disseminate information, not limited to our websites, email, sms, push notifications, influencers, social media postings, ticker tags, press releases, online interviews, podcasts, videos, audio ads, banner ads, native ads, and responsive ads. This compensation is a major conflict of interest in our ability to be unbiased regarding the subject of our reports and communications. Therefore, this communication should be viewed as a commercial advertisement only. We have not investigated the background of the parties who hired us, or of the profiled companies. Any non-compensated alerts are purely for the purpose of expanding our database for the benefit of our future financially compensated investor relations efforts.
The third parties paying for our services, the profiled company, or their affiliates likely wish to liquidate shares of the profiled company at or near the time you receive this communication, which has the potential to impact share prices, possibly significantly. Frequently companies profiled in our alerts experience a large increase in volume and share price during the course of investor relations marketing, which may end as soon as the investor relations marketing ceases.
We do not guarantee the timeliness, accuracy, or completeness of the information on our website / media webpage. The information in our website / media webpage is believed to be accurate and correct, but has not been independently verified and is not guaranteed to be correct. The information is collected from public sources, such as the profiled company’s website and press releases, but is not researched or verified in any way whatsoever to ensure the publicly available information is correct. Furthermore, Virtus Media often employs independent contractor writers who may make errors when researching information and preparing these communications regarding profiled companies. Independent writers’ works are double-checked and verified before publication, but it is possible for errors or omissions to take place during editing of independent contractor writer’s communications regarding the profiled company(s). You should assume all information in all of our communications is incorrect until you personally verify the information, and again are encouraged to never invest based on the information contained in our written communications. Our emails may contain forward-looking statements, which are not guaranteed to materialize due to a variety of factors.
Compensation: Pursuant to an agreement between Virtus Media LLC and JRZ Capital LLC, Virtus Media LLC has been hired by JRZ Capital LLC for a period beginning on 04/10/2024 and ending 05/03/2024 to publicly disseminate information about NASDAQ: VTGN via digital communications. We have been paid eighty three thousand nine hundred dollars USD. We have been paid an additional ten thousand dollars USD. Pursuant to an agreement between Virtus Media LLC and JRZ Capital LLC, Virtus Media LLC has been hired by JRZ Capital LLC for a period beginning on 9/23/2024 and lasting two weeks. We have been paid 10,000 USD. To date we have been paid one hundred three thousand nine hundred dollars USD to disseminate information about (NASDAQ:VTGN) via digital communications. We own zero shares of (NASDAQ:VTGN). Virtus media agrees to pay social media influencer #1 one thousand five hundred dollars USD and social media influencer #2 two thousand dollars USD and social media influencer #3 one thousand five hundred dollars USD and social media influencer #4 six hundred dollars USD and social media influencer #5 four hundred fifty dollars USD and social media influencer #6 three hundred fifty dollars USD and social media influencer #7 two hundred fifty dollars USD and social media influencer #8 one hundred fifty dollars USD and social media influencer #9 five hundred dollars USD and social media influencer #10 three hundred fifty dollars USD and social media influencer #11 two hundred dollars USD and social media influencer #12 six hundred dollars USD and social media influencer #13 four hundred fifty dollars USD and social media influencer #14 one thousand dollars USD and social media influencer #15 two thousand dollars USD and social media influencer #16 three thousand dollars USD and social media influencer #17 one thousand eight hundred dollars USD and social media influencer #18 one thousand seven hundred dollars USD and social media influencer #19 one thousand five hundred dollars USD and social media influencer #20 three hundred fifty dollars USD and social media influencer #21 three hundred fifty dollars USD and social media influencer #22 three hundred fifty dollars USD and social media influencer #23 three hundred fifty dollars USD and social media influencer #24 three hundred fifty dollars USD and social media influencer #25 four thousand dollars USD and social media influencer #26 two hundred dollars USD and social media influencer #27 two hundred fifty dollars USD and social media influencer #28 two thousand two hundred fifty dollars USD. Pursuant to a further agreement between Virtus Media LLC and JRZ Capital LLC, Virtus Media LLC has been hired by JRZ Capital LLC for a period beginning on 2025-02-12 and ending on 2025-03-12 to publicly disseminate information about NASDAQ: VTGN via digital communications. We have been paid sixty thousand dollars USD. Virtus Media LLC agrees to pay The Investing Authority LLC seven thousand dollars USD and social media influencer #1 two thousand five hundred dollars USD and social media influencer #2 one thousand dollars USD and social media influencer #3 two hundred fifty dollars USD and social media influencer #4 two hundred dollars USD.

